
Forum Co-Chairs
Robert Califf and Gregory Simon lead the 43rd Meeting of the Forum.

The Forum serves as a hub and a catalyst for nurturing new ideas and partnerships and offers a neutral space for stakeholders to advance critical policy discussions on biopharmaceutical innovation nationally and globally.
Message from the Co-Chairs
Robert Califf and Gregory Simon
Clinical trials remain a cornerstone of medical product development by providing the scientific evidence that proves or disproves concepts dur ng earlier stages of development about the safety and efficacy of medica products and by informing clinical care. At the same time, the clinical research enterprise faces continued and mounting pressures, strained from all sides by rising costs, an evolving regulatory and economic landscape, increasing clinical trial complexity, difficulties in the recruitment and retention of research participants, and a clinical research workforce that is under tremendous stress. These challenges cannot be overcome in isolation and will require collaboration among patients, providers, academia, industry, federal agencies, payers, nonprofit organizat ons, and funders.
Consider the past when health records were all on paper. Today, health systems serve millions of people and new technologies help improve our understanding of treatment and disease through the analysis of information from electronic health records and other data sources. We have entered an exciting time in biomedical research when clinical research and health care are at a critical juncture and the biological, physical, and digital spheres are merging. These opportunities hold great promise for advancing our understanding of health maintenance and prevention, disease progression, and for developing new therapies for patients.
The Forum on Drug Discovery, Development, and Translation (the Forum) of the National Academies of Sciences, Engineering, and Medicine (the National Academies) was created in 2005 by the National Academies’ Board on Health Sciences Policy to foster communication, collaboration, and action in a neutral setting on issues of mutual interest across the drug research and development (R&D) lifecycle. The Forum membership includes leaders from the National Institutes of Health (NIH), the U.S. Food and Drug Administration (FDA), the biopharmaceutical industry, academia, consortia, foundations, journals, and patient-focused and disease advocacy organizations.
In 2019, the Forum, in collaboration with the National Academies’ National Cancer Policy Forum, hosted a meeting on Updating Labels for Generic Oncology Drugs, which provided a venue to examine the challenges and opportunities for updating labeling for generic oncology drugs. In collaboration with the National Academies’ Forum on Neuroscience and Nervous System Disorders, National Cancer Policy Forum, and Roundtable on Genomics and Precision Health, the Forum hosted two workshops. One on Enhancing Scientific Reproducibility Through Transparent Reporting, which explored issues related to transparent reporting (e.g., the disclosure of the availability and location of data, materials, analysis, and methodology) to improve rigor and reproducibility in biomedical research. And one on Sharing Clinical Trial Data: Challenges and a Way Forward, which examined progress, lessons learned, and remaining gaps in clinical trial data sharing and reuse following the publication of the 2015 Institute of Medicine report Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk.
In 2020, more work is needed to spur biomedical innovation in a responsible manner and ensure that research is adequately powered to answer real questions about the safety and effectiveness of medical products. While we can point to important breakthroughs in understanding disease biology and the development of precision treatments, broader innovation is needed to address the major causes of disability and premature mortality. The executive and legislative branches of the federal government have continued to show bipartisan support for biomedical research, including ongoing funding for programs such as the BRAIN Initiative, the Precision Medicine Initiative, and the 21st Century Cures Act. Government funders and regulators of biomedical research, such as NIH and FDA, stand to receive continued infusions of funding and support for relevant programmatic priorities.
The Forum serves as a hub and a catalyst for nurturing new ideas and partnerships and offers a neutral space for stakeholders to advance critical policy discussions on biopharmaceutical innovation nationally and globally. Looking ahead to 2020, the Forum plans to focus on important issues such as examining the role of digital health technologies in drug development, improving drug innovation for older adult populations, and accelerating progress toward a clinical trials enterprise that is more efficient, effective, patient-centered, and integrated nto the health delivery system. We look forward to another groundbreaking and productive year for the Forum in 2020.


Robert Califf, co-chair and Gregory Simon, co-chair

Forum members at the 43nd Forum
meeting held in March 2019

Reflecting Back: Forum Activities in 2019
Forum Membership Meetings
In 2019, the Forum membership focused on diverse topics related to the Forum’s priorities, including issues for emerging biotech companies; transforming clinical trials through digital health; improving the drug development process through examining late-stage failures; real-world evidence and medical product development; harmonizing clinical trial site standards; data-sharing principles for nonprofit clinica trial funders; the science of patient input in medical product R&D; innovative models to spur translational research and drug discovery; artificial intelligence applications for drug discovery and development; and other policy updates relevant to drug discovery, development, and translation. To supplement the perspectives and expertise of its members, the Forum holds public workshops to focus attention on critical areas of drug development. Summaries of these meetings are disseminated to the public through the Forum website and published proceedings.
Workshops and Meetings
Updating Labels for Generic Oncology Drugs: A Meeting
The Forum, in collaboration with the National Academies’ National Cancer Policy Forum, hosted a public meeting, sponsored by FDA, on March 26, 2019, that convened expert stakeholders to discuss the challenges and opportunities for updating labels for generic oncology drugs. The meeting featured discussions about current regulatory requirements for labeling updates, review processes for clinical practice guidelines for oncology, and how outdated drug labeling can impact clinical care. Meeting participants considered potential sources of data and evidentiary standards to guide decision making for labeling updates, including indications for select patient populations (e.g., pediatric oncology), dosing, and adverse events.
Enhancing Scientific Reproducibility Through Transparent Reporting: A Workshop
In collaboration with the National Academies’ Forum on Neuroscience and Nervous System Disorders, National Cancer Policy Forum, and Roundtable on Genomics and Precision Health, the Forum convened a workshop on September 25–26, 2019, to examine the current state of transparency in reporting biomedical research (e.g., disclosure of the availability and location of data, materials, analysis, and methodology) and to explore the possibility of improving the harmonization of guidelines across journals and funding agencies so that biomedical researchers propose and report data in a consistent manner. Workshop participants discussed the challenges and opportunities for the harmonization of guidelines for transparent reporting throughout the biomedical research lifecycle. The agenda included a panel discussion on facilitating the development of consistent guidelines (e.g., a common set of minimal reporting standards) that could be applied across journals and funders to increase transparency in proposing and reporting biomedical research. This workshop was sponsored by NIH, Cell Press, The Lancet, and Nature Research.
Sharing Clinical Trial Data: Challenges and a Way Forward: A Workshop
In collaboration with the National Academies’ Forum on Neuroscience and Nervous System Disorders, National Cancer Policy Forum, and Roundtable on Genomics and Precision Health, the Forum hosted a public workshop to examine the progress and remaining gaps in clinical trial data sharing following the publication of the 2015 Institute of Medicine (IOM) report Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk. Workshop participants considered the value and potential risks/ costs of sharing clinical trial data for key stakeholders, including clinical trialists, sponsors, primary and secondary researchers, and patients. The agenda featured use cases from across public and private sectors when it comes to success, failure, lessons learned, and the value of data sharing. This workshop was sponsored by the Wellcome Trust.

Participants discuss actions stakeholders could take to improve transparent reporting of biomedical research during a 2019 workshop on Enhancing Scientific Reproducibility Through Transparent Reporting.

Looking Forward: Forum Activities in 2020
Forum Membership Meetings
The Forum membership will meet in March, July, and October 2020 to continue its discussions of key problems and strategies in the discovery, development, and translation of drugs. Forum workshop planning committees, working groups, and Action Collaboratives will convene to discuss and act on identified priority areas, including the following activities.
Workshops and Meetings
The Role of Digital Health Technologies in Drug Development
Digital health technologies (e.g., smartphone apps, wearable sensors, and other remote, sensor-based tools that combine hardware and software) have become increasingly available to consumers, providers, and researchers. They offer new opportunities to address critical challenges or “pain points,” better connect patients and health care providers, and incorporate patient input throughout the drug R&D lifecycle. In collaboration with the National Academies’ Roundtable on Genomics and Precision Health, the Forum will host a workshop on March 24, 2020, to discuss the challenges and opportunities in using digital health technologies to improve the probability of success in drug development. Workshop participants may consider key components for an evidence-based framework for applying digital health technologies toward drug R&D.
Drug Research and Development for Older Adult Populations
Despite the widespread recognition of the “graying of America,” and the need for health care among older adults, there is a dearth of information about the appropriate use of drugs in this population. Older adults are vastly underrepresent- ed in clinical trials. Yet, older adults have higher rates of comorbidities and polypharmacy than the general population and are the majority users of many medications. Additionally, age-related physiological and pathological changes, particularly for adults over age 80, can lead to significant di ferences in the pharmacokinetic and pharmacodynamics of a given drug compared to the general population. There is a void in evidence-based information for making informed decisions on how to best optimize care for older adults,particularly those over age 80. In collaboration with the National Academies’ Forum on Aging, Disability, and Independence and the National Academies’ National Cancer Policy Forum, the Forum will host a workshop to discuss the challenges and opportunities in drug R&D for the over age 65 and over age 80 populations, explore barriers that impede safety and efficacy studies in these populations, and share lessons learned and best practices for better understanding the clinical pharmacology for older adults.
Envisioning a Transformed Clinical Trials Enterprise for 2030
Clinical trials research has changed dramatically over the past decade. The biological, physical, and digital spheres are merging; clinical research and health care are at a critical juncture; and new technologies enable the collection of data in real-world settings. These opportunities hold great promise for advancing our understanding of health maintenance and prevention, disease progression, and developing new therapies for patients. At the same time, the clinical research enterprise faces continued and mounting pressures, strained from all sides by rising costs, an evolving regulatory and economic landscape, increasing clinical trial complexity, difficulties in the recruitment and retention of research participants, and a clinical research workforce that is under tremendous stress. Some, but not all, of these challenges and opportunities were predicted in the 2011 Forum- hosted workshop Envisioning a Transformed Clinical Trials Enterprise in the United States: Establishing an Agenda for 2020. There is now a need for stakeholders from across the clinical research lifecycle, including patients, caregivers, regulators, practitioners, and researchers, to examine the challenges and setbacks, lessons learned, and advances from the past decade, and consider the challenges and opportunities that may be coming over the next 10 years. Workshop discussions will focus broadly on how to better integrate community practice and clinical trials, improve patient engagement in clinical trials, align cultural and financial incentives, and support the clinical trials infrastructure. The workshop will provide a venue to discuss goals and key priorities for advancing a clinical trials enterprise that is more efficient, effective, patient-centered, and integrated into the health delivery system of 2030.

Participants at the 2019 workshop on Sharing Clinical Trial Data: Challenges and a Way Forward
Action Collaboratives
Action collaboratives are ad hoc, participant-driven activities associated with the Forum that foster collaboration and information sharing among Forum members and external thought leaders and stakeholders. Action collaboratives engage experts with similar interests and responsibilities to analyze in-depth, high-priority issues and to advance the identified goals of the Forum and recommendations highlighted in National Academies’ Consensus Study Reports.
Advancing the Science of Patient Input in Medical Product R&D
There is growing momentum to incorporate patient input into medical product R&D and regulatory decision-making processes. Converting traditionally anecdotal patient input to rigorous, credible evidence for use by a broad range of stakeholders—including academic and clinical researchers, medical product developers, patient/disease advocacy groups, and regulatory decision makers—could better align medical product development and regulation with patient perspectives on disease experience, burden, management, and treatment. Many efforts have been launched to advance a science of patient input. However, there is a need to identify key gaps in the knowledge base and other barriers that impede progress and to develop an approach for addressing them. This action collaborative launched in 2017 to foster the development of a strategic research framework to advance the science of patient input. During the first phase of work, collaborative participants catalogued current efforts and progress in the science of patient input and identified gaps in the knowledge base and other barriers that impede progress. In the second phase, collaborative participants laid the groundwork for a public workshop hosted by the Forum on May 9, 2018, titled Advancing the Science of Patient Input in Medical Product R&D: Towards a Research Agenda. Topics raised during this discussion-based workshop will help inform the third phase, which will focus on the development of a research framework for advancing the science of patient input.
Clinical Trial Site Standards Harmonization
Mobilized in 2012, this action collaborative set out to explore opportunities to improve clinical trial site functioning with the goal of increasing productivity in medical product development. To date, the collaborative has drawn together a group of diverse stakeholders for four in-person meetings (December 2012, August 2013, March 2014, and September 2018). Collaborative participants published a National Academy of Medicine Discussion Paper in October 2017, which summarizes their perspectives on a proposed launching point for harmonizing the requirements applied to clinical trial sites and the development of standards. In 2018, the collaborative collected, analyzed, and assessed a set of baseline site standards necessary for launching the majority of phase II, III, and IV clinical trials, and which could be considered for broad application across clinical trial sites by research sponsors.
Improving the Drug Development Process Through Examining Late-Stage Failures
The development of new drugs is inherently complex and costly, with sources documenting up to $2.6 billion and more than 9 years in investment to bring a new treatment to market. The high cost is driven, at least in part, by the failure rate for new compounds entering late-stage development, but also encompasses opportunity costs due to time and resources not spent on developing an alternate treatment candidate, as well as investment in clinical trial infrastructure. Most importantly, these failures have an impact on patients—those who participate in the clinical trials associated with the testing of a failed drug candidate and those who will not benefit from having access to treatments that could have been developed instead. The goal of this action collaborative is to examine the contributing factors to late-stage failures and develop a set of key considerations for stakeholders to improve the probability of success in late-stage product development. In 2018, collaborative participants conducted structured, qualitative interviews with subject-matter experts; analyzed and synthesized responses from these interviews; considered information compiled from a literature survey; and held a meeting on October 3, 2018, to discuss and inform the key considerations. In 2019, collaborative participants will continue to prepare and publish a written summary of the key considerations and points identified by this activity.
Sharing Clinical Trial Data
Sharing clinical trial data can help facilitate more efficient and effective development of better medicines, diagnostics, and procedures for the ultimate benefit of patients. At the same time, sharing data presents risks, burdens, and challenges that need to be addressed by a broad set of stakeholders. These opportunities and challenges were laid out in the IOM report Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk, which calls on stakeholders to foster a culture of sharing and offers a blueprint for action within and across sectors. Four National Academies forums and roundtables, including the Forum, provided momentum and a framework for initiating the consensus study that produced the report and continue to work together to support coordination and collaboration among stakeholders engaged in data-sharing initiatives through convening and other activities.
This action collaborative, in collaboration with Brigham and Women’s Hospital and Harvard University’s Multi-Regional Clinical Trials Center, helped inform the establishment of Vivli—a global data repository, Cloud-based analytics platform, and in-depth search engine. The public launch of Vivli took place at the National Academies on July 19, 2018. The action collaborative also worked to establish data-sharing goals for nonprofit funders of clinical trials. On November 30, 2017, the collaborative convened a meeting of leaders of nonprofit disease advocacy organizations, philanthropic organizations and foundations, federal government, academia, industry, and others to review a draft of the Statement of Data-Sharing Goals for Nonprofit Funders of Clinical Trials, discuss the associated risks and challenges with implementing the goals, and explore the next steps for operationalizing them. In 2019, collaborative participants will continue to work toward publication of this statement of data-sharing goals.

Forum members (from left to right) Ross McKinney, Jr., Anantha Shekhar, Bernard Munos, and Ann E. Taylor sharing their thoughts at the July 2019 Forum meeting.

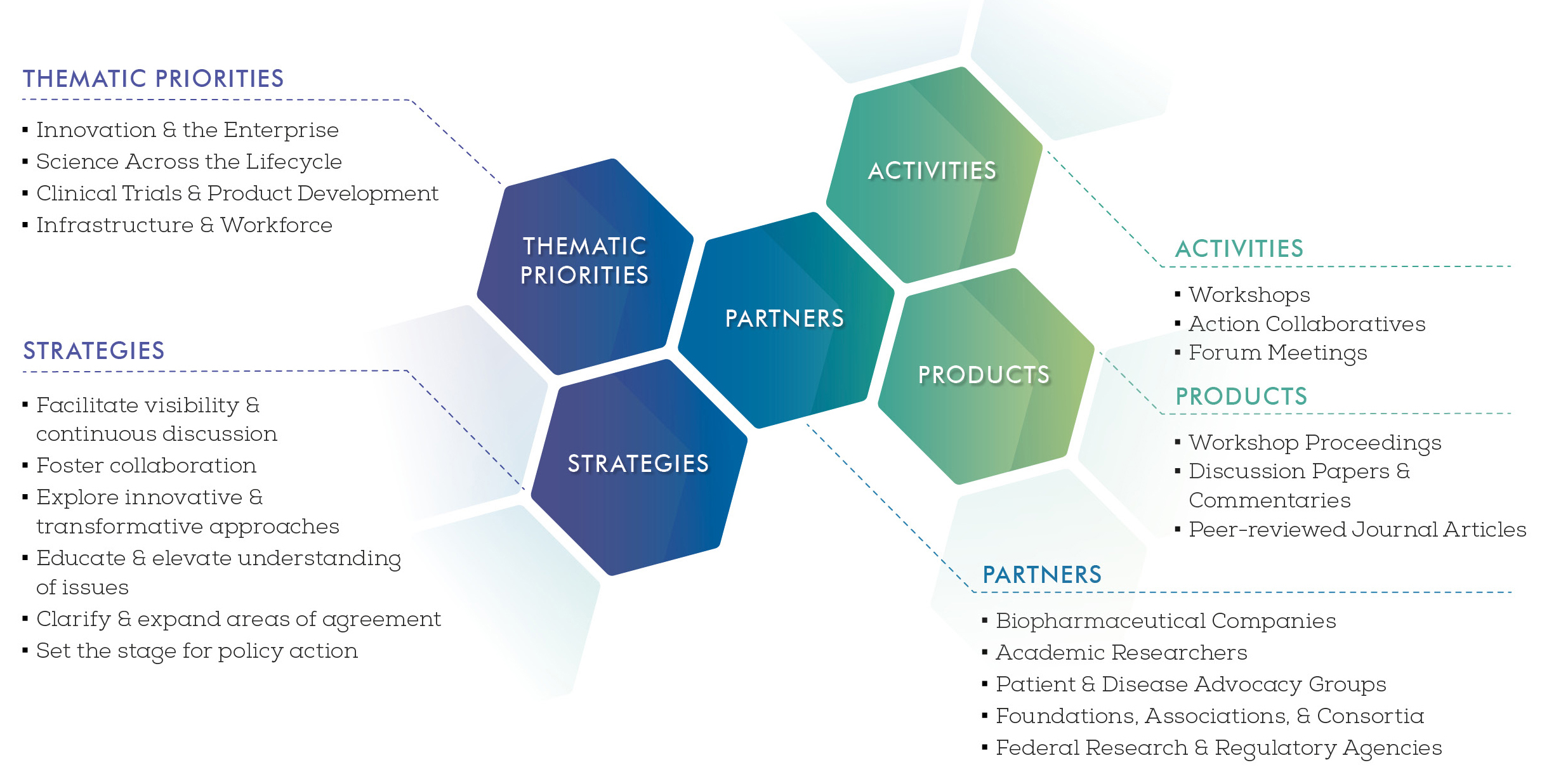
Forum Themes and Priorities
The Forum seeks to address the key challenges and opportunities in the discovery, development, and translation of new therapeutics for patients, covering the full continuum from basic discovery to the approval and adoption of new therapies into clinical practice. As an overarching and cross-cutting theme, the Forum fosters innovative efforts to identify and highlight potentially breakthrough ideas and visionary approaches to the drug development and translational science enterprise of the future. The Forum identified four core components across the R&D lifecycle, which serve as thematic pillars to frame the Forum’s focus areas and activities: (1) Innovation and the Drug Research and Development Enterprise; (2) Science Across the Biomedical Research Lifecycle (Basic, Translational, and Regulatory Sciences); (3) Clinical Trials and Medical Product Development; and (4) Infrastructure and Workforce for Advancing Drug Discovery, Development, and Translation.
Innovation and the Drug Research and Development Enterprise
Despite exciting scientific advances, the path from basic science to new therapeutics faces challenges on many fronts. Innovative paradigms are needed to bridge the ever-widening gap between scientific discoveries and the translation of those discoveries into life-changing medications. There is increasing recognition of the need for new models and methodologies to advance drug discovery, development, and translational science. The Forum serves as a hub and a catalyst for nurturing new ideas, collaborations, and partnerships and offers a neutral space for stakeholders to help chart a course through the turbulent forces of disruptive innovation and advance critical policy discussions on biopharmaceutical R&D nationally and globally.
Science Across the Biomedical Research Lifecycle
Revolutionary advances in biomedical research and technology applications present new and exciting opportunities for the discovery and development of new therapies for patients. At the same time, key gaps remain in our knowledge about the science, technology, and methodologies needed to support the research that underpins drug discovery and development. The Forum provides a neutral venue to focus attention on key scientific issues across the biomedical research lifecycle and facilitate the exploration of new and more patient-centric approaches to spur innovation. The Forum continues to support activities that contribute toward the defining, establishment, and refinement of biomedical and regulatory science and other critical aspects of drug discovery and medical product development.
Clinical Trials and Medical Product Development
Clinical research provides a critical link between the laboratory bench and patients’ bedside when it comes to developing new therapeutics and advancing understanding of health maintenance and prevention and disease progression. However, the clinical research enterprise faces continued and mounting pressures, strained from all sides by rising costs, an evolving regulatory and economic landscape, increasing clinical trial complexity, difficulties in the recruitment and retention of research participants, and a clinical research workforce that is under tremendous stress. Collaborative, cross-sector approaches that engage patients, providers, academia, industry, federal agencies, payers, nonprofit organizations, and funders can help to effectively address the key challenges and to develop systems-based solutions.
Infrastructure and Workforce for Advancing Drug Discovery, Development, and Translation
Considerable opportunities remain for the improvement and enhancement of the infrastructure—the organizational framework, systems, workforce, and other resources—that supports the drug discovery and development enterprise. The fields of drug discovery and development, clinical research, and clinical practice are intersecting, cross-cutting, and multidisciplinary. However, career paths in these fields are difficult to pursue, training may be lacking, and career opportunities may be limited. The Forum has considered workforce needs as foundational for the advancement of drug discovery, development, and translation and has focused substantial attention on these issues and fostered the development of strategies to improve the discipline of innovative regulatory science through the development of a robust workforce.
Sponsors
(as of December 31, 2019)
Financial support for the Forum is derived from government agencies, industry sponsors, private foundations, and nonprofit associations.
Government
Center for Drug Evaluation and Research, FDA
National Cancer Institute, NIH
National Center for Advancing Translational Sciences, NIH
National Institute of Allergy and Infectious Diseases, NIH
National Institute of Mental Health, NIH
National Institute of Neurological Disorders and Stroke, NIH
Office of Science Policy, NIH
Industry
AbbVie Inc.
Amgen Inc.
AstraZeneca
Eli Lilly and Company
GlaxoSmithKline
Johnson & Johnson
Merck & Co., Inc.
Sanofi
Takeda Pharmaceuticals
Private Foundations
Burroughs Wellcome Fund
Nonprofit Organizations
Association of American Medical Colleges
Critical Path Institute
FasterCures
Foundation for the National Institutes of Health
Friends of Cancer Research
New England Journal of Medicine
Members
(as of December 31, 2018)
Forum membership includes a diverse range of stakeholders from multiple sectors, including government, biopharmaceutical industry, biomedical research funders and sponsors, academia, foundations, consortia, disease advocacy, and patient-focused groups.
Robert Califf (Co-Chair)
Verily Life Sciences
Gregory Simon (Co-Chair)
Kaiser Permanente Washington Health Research Institute and University of Washington
Christopher Austin
National Center for Advancing Translational Sciences, NIH
Linda Brady
National Institute of Mental Health, NIH
Tanisha Carino
FasterCures
(until October 2019)
Barry Coller
The Rockefeller University
Thomas Curran
Children’s Mercy, Kansas City
Richard Davey
National Institute of Allergy and Infectious Diseases, NIH
James Doroshow
National Cancer Institute, NIH
Jeffrey Drazen
New England Journal of Medicine
Steven Galson
Amgen Inc.
Carlos Garner
Eli Lilly and Company
Julie Gerberding
Merck & Co., Inc.
Anne Heatherington
Takeda Pharmaceuticals
Lynn Hudson
Critical Path Institute
Deborah Hung
Broad Institute of the Massachusetts Institute of Technology and Harvard Medical School
S. Claiborne Johnston
Dell Medical School, The University of Texas at Austin
Esther Krofah
FasterCures
(as of November 2019)
 *Consortia, Foundations, Journals, Patient-Focused/Disease Advocacy Organizations
*Consortia, Foundations, Journals, Patient-Focused/Disease Advocacy Organizations
Ross McKinney, Jr.
Association of American Medical Colleges
Joseph Menetski
Foundation for the National Institutes of Health
Bernard Munos
InnoThink Center for Research in Biomedical Innovation
Kelly Rose
Burroughs Wellcome Fund
Joseph Scheeren Critical Path Institute (as of October 2019)
Rob Scott
AbbVie Inc.
(as of August 2019)
Michael Severino
AbbVie Inc.
(until August 2019)
Anantha Shekhar
Indiana University School of Medicine
Ellen Sigal
Friends of Cancer Research
Lana Skirboll
Sanofi
Amir Tamiz
National Institute of Neurological Disorders and Stroke, NIH
Ann Taylor
AstraZeneca
Pamela Tenaerts
Clinical Trials Transformation Initiative
John Wagner
Takeda Pharmaceuticals
(until March 2019)
Joanne Waldstreicher
Johnson & Johnson
Carrie Wolinetz
Office of Science Policy, NIH
Alastair Wood
Vanderbilt University
Janet Woodcock
Center for Drug Evaluation and Research, FDA
Timeline
2019 | March 6–7 Forum Meeting #42 | March 26 Meeting: Updating Labels for Generic Oncology Drugs (in collaboration with the National Cancer Policy Forum) | July 30–31 Forum Meeting #43 | September 25–26 Workshop: Enhancing Scientific Reproducibility Through Transparent Reporting (in collaboration with the Forum on Neuroscience and Nervous System Disorders, the National Cancer Policy Forum, and the Roundtable on Genomics and Precision Health) | October 23–24 Forum Meeting #44 | November 18–19 Workshop: Sharing Clinical Trial Data: Challenges and a Way Forward (in collaboration with the Forum on Neuroscience and Nervous System Disorders, the National Cancer Policy Forum, and the Roundtable on Genomics and Precision Health)
2018 | March 6–7 Workshop Series: Examining the Impact of Real-World Evidence on Medical Product Development—Workshop 2: Practical Approaches | March 19–20 Forum Meeting #39 | May 9 Workshop: Advancing the Science of Patient Input in Medical Product R&D—Towards a Research Agenda | July 17–18 Workshop Series: Examining the Impact of Real-World Evidence on Medical Product Development—Workshop 3: Application | July 18 Forum Meeting #40 | October 3–4 Forum Meeting #41 | November 28–29 Workshop: Virtual Clinical Trials—Challenges and Opportunities
2017 | March 8 Workshop: Enabling Precision Medicine: The Role of Genetics in Clinical Drug Development (in collaboration with the Genomics Roundtable) | March 9 Forum Meeting #36 | July 10-11 Forum Meeting #37 | September 19-20 Workshop Series: Examining the Impact of Real-World Evidence on Medical Product Development-Workshop 1: Incentives | October 24-25 Forum Meeting #38
2016 | March 22 Workshop: Deriving Drug Discovery Value from Large-Scale Genetic Bioresources (in collaboration with the Genomics Roundtable) | March 23 Forum Meeting #33 | July 19-20 Forum Meeting #34 | October 18 Forum Meeting #35 | October 19 Workshop: Real-World Evidence Generation and Evaluation of Therapeutics | December 12-13 Workshop: The Drug Development Paradigm in Oncology (in collaboration with the National Cancer Policy Forum)
2015 | January 20-21 Workshop: Financial Incentives to Support Unmet Medical Needs for Nervous System Disorders (in collaboration with the Neuroscience Forum) | March 17-18 Forum Meeting #30 | March 26-27 Workshop: Rapid Medical Countermeasure Response to Infectious Diseases: Enabling Sustainable Capabilities Through Ongoing Public- and Private-Sector Partnerships (in collaboration with the Medical Preparedness Forum) | June 23-24 Forum Meeting #31 | October 20 Workshop: Advancing the Discipline of Regulatory Science for Medical Product Development: An Update on Progress and a Forward-Looking Agenda | October 21 Forum Meeting #32
2014 | February 12 Workshop: Characterizing and Communicating Uncertainty in the Assessment of Benefits and Risks of Pharmaceutical Products (Day 1) | March 3-4 Forum Meeting #27 | May 12 Workshop: Characterizing and Communicating Uncertainty in the Assessment of Benefits and Risks of Pharmaceutical Products (Day 2) | June 10-11 Forum Meeting #28 | October 7-8 Forum Meeting #29
2013 | January 15 Workshop Summary Report Release: Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis | January 16-18 Workshop: The Global Crisis of Drug-Resistant Tuberculosis and Leadership of the BRIGS Countries (Beijing, China) | February 12 Forum Meeting #24 | February 13-14 Workshop: International Regulatory Harmonization Amid Globalization of Biomedical Research and Medical Product Development | June 3 Forum Meeting #25 | October 28-29 Forum Meeting #26
2012 | March 13-14 Forum Meeting #21 | June 4-5 Workshop: Maximizing the Goals of the Cures Acceleration Network to Accelerate the Development of New Drugs and Diagnostics | June 5 Forum Meeting #22 | July 31-August 1 Workshop: Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis | October 4-5 Workshop: Sharing Clinical Research Data (in collaboration with the Neuroscience Forum, National Cancer Policy Forum, and Genomics Roundtable) | October 23-24 Forum Meeting #23 | November 26-27 Workshop: Large Simple Trials and Knowledge Generation in a Learning Health System (in collaboration with the Leadership Consortium for a Value & Science-Driven Health System)
2011 | March 28 Forum Meeting #18 | March 29-30 Workshop: Advancing Regulatory Science for Medical Countermeasure Development (in collaboration with the Medical Preparedness Forum) | April 18-19 and 21 Workshop: Facing the Reality of Multidrug-Resistant Tuberculosis: Challenges and Potential Solutions (New Delhi, India) | June 27-28 Workshop: Public Engagement and Clinical Trials: New Models and Disruptive Technologies (in collaboration with the Mount Sinai School of Medicine) | June 28-29 Forum Meeting #19 | July 12 Workshop Summary Report Release: The Emerging Threat of Multidrug-Resistant Tuberculosis: Global and Local Challenges and Solutions (Durban, South Africa) | September 20-21 Workshop: Strengthening a Workforce for Innovative Regulatory Science in Therapeutics Development | October 4-5 Forum Meeting #20 | November 7-8 Workshop: Envisioning a Transformed Clinical Trials Enterprise in the United States: Establishing an Agenda for 2020 | November 15 Workshop Summary Report Release: The New Profile of Drug-Resistant Tuberculosis: A Global and Local Perspective (Moscow, Russia)
2010 | February 23-24 Workshop: The Public Health Emergency Medical Countermeasures Enterprise (in collaboration with the Medical Preparedness Forum) | February 26 Workshop: Building a National Framework for the Establishment of Regulatory Science for Drug Development | March 3-4 Workshop: The Emerging Threat of Multidrug-Resistant Tuberculosis (Pretoria, South Africa) | April 29-30 Forum Meeting #15 | May 26-27 Workshop: The New Profile of Drug-Resistant Tuberculosis (Moscow, Russia) | August 5 Forum Meeting #16: Conflict of Interest | October 29 Forum Meeting #17: Administrative and Regulatory Inefficiencies in Clinical Trials
2009 | February 23 Capitol Hill Briefing: Growing Threat of Drug-Resistant Tuberculosis | March 13 Discussion Series: FDA Community Update on Personalized Medicine and the Genetic Basis of Adverse Events | April 27 Workshop: Streamlining Clinical Trial and Material Transfer Negotiations | April 27-28 Forum Meeting #12 | July 10 Symposium: Drug Regulation with FDA Commissioner Peggy Hamburg and Forum Meeting #13 | September 2 Discussion Series: FDA Community Update: Improving the Science of Drug Safety | October 7-8 Workshop: Transforming Clinical Research in the United States | October 15-16 Forum Meeting #14
2008 | February 20-21 Forum Meeting #9 | February 20 Discussion Series: Comparative Effectiveness | April 21 Discussion Series: Science at FDA: Challenges and Opportunities | June 23 Workshop: Breakthrough Business Models: Drug Development for Rare and Neglected Diseases and Individualized Therapies | June 24 Forum Meeting #10 | October 24 Workshop: Assessing and Accelerating Development of Biomarkers for Drug Safety | November 2-3 Forum Meeting #11 | November 5 Workshop: Addressing the Threat of Drug-Resistant Tuberculosis: A Realistic Assessment of the Challenge
2007 | March 12 Symposium: The Future of Drug Safety: Challenges for the FDA | April 23-24 Workshop: Emerging Safety Science | April 24 Forum Meeting #7 | September 14 Discussion Series: From Patient Needs to New Drug Therapies: Can We Improve the Pathway? | October 15-16 Forum Meeting #8 | November 30 Discussion Series: A Conversation with Tony Fauci
2006 | March 28-29 Forum Meeting #4 | May 30-31 Workshop: Understanding the Benefits and Risks of Pharmaceuticals | June 13 Workshop: Addressing the Barriers to Pediatric Drug Development | June 27-28 Forum Meeting #5 | October 24-25 Forum Meeting #6
2005 | Forum on Drug Discovery, Development, and Translation founded | March 23-24 Forum Meeting #1 | June 29-30 Forum Meeting #2 | September 8-9 Forum Meeting #3 | November 3-4 Workshop: Adverse Drug Event Reporting: The Roles of Consumers and Health-Care Professionals
2002-2004 | Clinical Research Roundtable, predecessor to the Forum
