B
Iron Deficiency Anemia: A Synthesis of Current Scientific Knowledge and U.S. Recommendations for Prevention and Treatment
Peter R. Dallman
Iron deficiency anemia is a relatively common nutritional problem in the United States, particularly among infants, adolescents, and women of childbearing age. Its prevention deserves a high priority because iron deficiency anemia has serious consequences, yet its prevalence can be substantially reduced at modest cost. There has been great progress in preventing iron deficiency anemia among infants and children, but the prevalence among pregnant women of childbearing age remains high. The purpose of this appendix is to provide a brief review of the characteristics of iron deficiency anemia and to review recent guidelines for its prevention in primary health care settings.
This appendix provided background information for the Committee on the Prevention, Detection, and Management of Iron Deficiency Anemia Among U.S. Children and Women of Childbearing Age of the Food and Nutrition Board, Institute of Medicine. It also incorporated revisions and additions suggested after review of the paper by the committee, but does not indicate universal concurrence with its content. The committee then developed summary guidelines for children, nonpregnant women of childbearing age, and pregnant women that are contained in the main report.
Review of Knowledge
Metabolism and Physiology
Iron in the Body
The total amount of iron in the body of an adult woman averages 2.3 g (Bothwell et al., 1979), about the weight of a dime. Figure B-1 shows the distribution of this iron, which is similar in women of reproductive age and in children. An average of about 85 percent of total body iron can be classified as essential because it serves well-defined physiologic functions. Essential iron
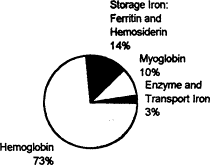
Figure B-1 Distribution of iron in women of childbearing potential (total body iron, 2.3 g—the weight of a dime).
Source: Values are from Table 1, p. 2, in Bothwell and Charlton (1981).
compounds include hemoglobin, which accounts for about three-quarters of total body iron and functions in the transport of oxygen from the lungs to tissues. Because hemoglobin circulates in the blood and accounts for a large proportion of essential body iron, its concentration often best reflects iron status. Other essential iron compounds include myoglobin, the red iron protein of muscle, and the mitochondrial iron proteins, which are essential for the oxidative production of cellular energy in the form of adenosine triphosphate. Iron deficiency is not associated with physiologic impairment until the production of essential iron compounds is diminished (Dallman, 1986).
A second category of iron compounds is referred to as storage iron. Storage iron compounds include ferritin and hemosiderin, which are present primarily in the liver, spleen, and bone marrow. They serve as a reserve that ensures an adequate supply of iron for the production of essential iron compounds, and they maintain body iron homeostasis by regulating the amount of iron absorbed from the diet. Storage iron is less abundant in women and children than in men: about 14 percent of total body iron, on average (Figure B-l), and about 25 percent respectively. The serum ferritin concentration provides an estimate of storage iron reserves.
Iron Homeostasis
Body Iron Regulation
Body iron is regulated primarily by modifying the percentage of food iron that is absorbed. Among healthy, nonpregnant women, body iron remains relatively stable, because the amount of iron absorbed each

Figure B-2 Iron balance in women of childbearing potential. Source: Based on Bothwell et al. (1979).
day is roughly equivalent to the amount of iron lost (Figure B-2) and is less than 0.05 percent of total body iron (Bothwell et al., 1979). Iron homeostasis is normally maintained because iron absorption is inversely proportional to the mount of storage iron. When storage iron decreases, as it does during pregnancy or rapid growth, iron absorption increases (Figure B-3). This homeostatic adaptation is greatest with diets containing high levels of available iron (Cook, 1990). Low iron stores per se indicate that an individual is vulnerable to developing iron deficiency anemia, but as long as the production of essential iron remains intact, there are no known physiologic handicaps from having low iron reserves (Dallman, 1986).
Iron Loss and Absorption Differences between Women and Men
Women have greater iron losses and absorb a greater percentage of iron from food than do men. During their childbearing years, women typically have less storage iron than men primarily because of menstrual blood loss (Bothwell et al., 1979). They compensate by absorbing, on average, about twice as much iron from the diet as men, 12 versus 6 percent (Table B-1).
Average menstrual blood loss is about 30 ml/month (Hallberg et al., 1966), but 10 percent of women regularly lose more than 80 ml/month (Figure B-4) and are likely to become anemic because their iron loss is usually greater than that which can be compensated for by increased absorption of iron from the
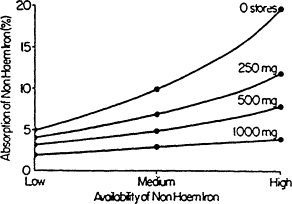
Figure B-3 Non-heme iron absorption from three different types of diets as the percent absorption of non-heme iron by individuals with no body iron stores and with 250-, 500-, and 1,000-mg iron stores. As iron stores decrease, the percentage of iron absorbed increases, helping to maintain homeostasis. This compensatory mechanism is less effective with diets of low iron bioavailability (common in developing countries) than with diets with medium and high levels of iron bioavailability, which are more typical in the United States. A daily diet of low iron bioavailability is one containing fewer than 30 g of meat, poultry, or fish (lean, raw weight) or less than 25 mg of ascorbic acid. The comparable figures for a diet of medium iron bioavailability are 30 to 90 g of meat, poultry, or fish or 25 to 75 mg of ascorbic acid, whereas a diet of high iron bioavailability is one containing more than 90 g of meat, poultry, or fish or more than 75 mg of ascorbic acid. Alternatively, it is one containing 30 to 90 g of meat, poultry, or fish plus 25 to 75 mg of ascorbic acid. SOURCE: Data from Monsen and coworkers (197g), in Bothwell et al. (1979).
diet. Unfortunately, such women are typically unaware of their high levels of blood loss. Consequently, the most practical way to identify them is by screening for anemia as part of a periodic health maintenance checkup (LSRO, 1991).
Menstrual blood loss varies with some methods of contraception (Figure B-5), roughly decreasing to half with oral contraceptives (the pill) and doubling with intrauterine devices (IUDs) (Bothwell and Charlton, 1981), Thus, inquiring about the method of contraception helps to predict the risk of iron deficiency; the risk is greatest in women who use an IUD.
Iron Needs During Pregnancy
Pregnancy imposes increased iron needs for the growth of the fetus and for expansion of maternal blood volume (Hallberg, 1988; IOM, 1990a) (Figure B-6). Even women who are not iron deficient at the beginning of pregnancy (on the basis of the hemoglobin concentration) are at risk of developing an iron-responsive depression in hemoglobin concentration in the third trimester unless they receive supplemental iron (Table B-2). Among women who are already iron deficient when they become pregnant, the severity of the deficiency will usually increase as pregnancy progresses unless they take an iron supplement.
TABLE B-1 Iron Balance in Women Compared with That in Men
|
Iron Parameter |
Women |
Men |
|
Total body iron, g |
2.3 |
3.5 |
|
Storage iron, g |
0.3 |
1.0 |
|
Food iron, mg/day |
11 |
15 |
|
Iron absorption, percent |
12 |
6 |
|
Iron absorption, mg/day |
1.3 |
0.9 |
|
Iron loss, mg/day |
1.3 |
0.9 |
|
SOURCE: Based primarily on data in Bothwell et al. (1979). |
||

Figure B-4 Frequency distribution of menstrual blood loss. Although the mean menstrual blood loss is about 30 ml/month, about 10 percent of women lose more than 80 ml/month. SOURCE: Data from Hallberg et al. (1966), adapted from Bothwell et al. (1979).

Figure B-5 Menstrual blood loss by method of contraception as mean ± standard deviation menstrual blood loss in three groups of women. The control group comprised normal women, the pill group comprised normal women taking the combination variety of oral contraceptives, and the IUD group comprised women using intrauterine devices (IUDs). Source: Figure from Bothwell and Charlton (1981).

Figure B-6 Schematic representation of the need for absorbed iron during pregnancy. Iron requirements increase markedly during the second and third trimesters. Source: From Bothwell et al. (1979).
TABLE B-2 Effects of Iron Supplementation on Mean Hemoglobin Concentration in Late Pregnancy
|
Dose of Elemental Irona |
Number of Subjects |
Hemoglobin, g/dl, at 35-36 weeks of Gestation |
Reference |
|||
|
Supplemented |
Controls |
Supplemented |
Controls |
Differenceb |
||
|
30 mg/day as ferrous |
49 |
46 |
12.4 |
11.4 |
1.0 |
Chanarin and Rothman, 1971 |
|
fumaratec |
|
|
|
|
|
|
|
100 mg, twice daily, with meals, sustained release |
24 |
26 |
12.4 |
11.4 |
1.0 |
Svanberg et al., 1976 |
|
100 mg, twice daily, sustained release |
16 |
16 |
12.7 |
11.0 |
1.7 |
Puolakka et al., 1980 |
|
65 mg (+ 350 µg of folate) |
21 |
24 |
12.7 |
11.0 |
1.6 |
Taylor et al., 1982 |
|
200 mg/day |
22 |
23 |
12.6 |
11.3 |
1.3 |
Romslo et al., 1983 |
|
105 mg, sustained release, at breakfast |
21 |
23 |
12.6 |
12.2 |
0.4 |
Wallenburg and van Eijk, 1984 |
|
65 mg as part of multivitamin-mineral supplement after meals |
16 |
13 |
12.4 |
11.4 |
1.0 |
Dawson and McGanity, 1987 |
|
a Ferrous sulfate, unless otherwise stated. b All differences were statistically significant except for Wallenburg and van Eijk (1984). c Doses of 60 and 120 mg did not result in higher hemoglobin values. SOURCE: From IOM (1990b). |
||||||
Iron Needs of Infants
Among infants, iron needs are primarily for growth. A high hemoglobin concentration at birth and abundant neonatal iron stores protect most term infants against iron deficiency until 4 months of age (Dallman, 1988). Indeed, total body iron scarcely changes during this period because of the physiologic decline in hemoglobin concentration; iron stores also diminish by 4 months of age (Figure B-7). Term infants are at the greatest risk of developing iron deficiency between 4 and 12 months of age and subsequently, when the iron needs for rapid growth must be supplied by the diet. At 1 year of age, for example, iron absorption is about four times greater than excretion, the difference being used for growth (Figure B-8). The risk of developing iron deficiency anemia during this period depends largely on the diet (Penrod et al., 1990; Pizarro et al., 1991; Tunnessen and Oski, 1987). Although iron deficiency anemia is rare in infants receiving iron-fortified formula, it is common in those fed unfortified formula or cow's milk (Figure B-9). Cow's milk not only has an extremely low concentration of iron but it also results in increased fecal blood loss (Ziegler et al., 1990) (Figure B-10). Furthermore, the higher calcium content of cow's milk compared with that of breast milk contributes to poor iron absorption (Hallberg et al., 1992). Exclusively breastfed infants may also develop iron deficiency, but only after about 6 months of age (Calvo et al., 1992; Duncan et al., 1985; Pizarro et al., 1991; Siimes et al., 1984), if they are not given an iron supplement (Figure B-9).
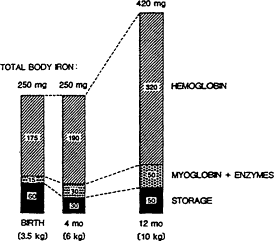
Figure B-7 Changes in body iron during infancy. There is little change in total body iron between birth and 4 months of age. In contrast, total body iron increases markedly during later infancy. The high iron needs from 4 to 12 months of age help to explain why the risk of iron deficiency is greatest during this period. Source: Dallman (1988).
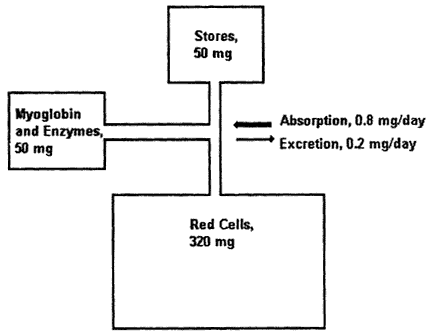
Figure B-8 Iron metabolism in the 1-year-old infant. Iron absorption must exceed iron loss to allow growth; however, daily iron absorption and loss, even in infancy, are normally a minute percentage of total body iron. MB + ENZ = myoglobin and enzyme iron. Source: Reproduced with minor modifications from Dallman (1988), with permission from Hanley & Belfus.
In the United States, there has recently been a marked decline in the prevalence of iron deficiency anemia among infants and young children (Yip et al., 1987a,b). This improvement is attributable to concurrent changes in infant feeding practices that would be expected to improve iron nutrition, including less use of cow's milk in the first year of life, more use of iron-fortified formulas, and less use of low-iron formulas (Fomon, 1987). Iron absorption studies suggest (Fomon et al., 1989) and clinical trials indicate (Walter et al., 1993) that iron-fortified infant cereals also play a significant role in preventing iron deficiency anemia.
Low-birth-weight infants may become iron deficient after 2 months of age and possibly earlier unless they are given an iron supplement (Lundström et al., 1977; Siimes et al., 1984) (Figure B-11). Their iron needs are greater because of their lower neonatal stores, a more rapid relative growth rate, and often, blood loss resulting from the increased number of laboratory studies that their early care may require. For low-birth-weight infants fed human milk, supplemental iron is recommended to start at about 2 weeks of age at a dose of 2 to 3 mg of elemental iron per kg/day (AAP, CON, 1985). Infants fed iron-fortified formula usually obtain sufficient amounts of iron to make an additional supplement unnecessary.
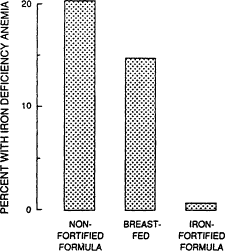
Figure B-9 Iron deficiency anemia among 9-month-old children who have been fed different diets. Source: From Pizzaro et al. (1991).
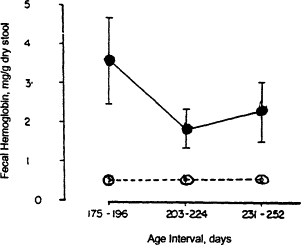
Figure B-10 The fecal hemoglobin concentrations of infants fed formula (•) and infants fed cow's milk (o) after 168 days of age. Early feeding of cow's milk to infants results in increased fecal blood loss. Bars indicate standard errors. Source: Adapted from Ziegler et al. (1990).

Figure B-11 Iron deficiency anemia among low-birth-weight infants. Low-birth -weight infants weighing 1,000 to 2,000 g are likely to develop iron deficiency anemia after 2 months of age if not given iron supplements (o). The supplemented infants (•) received a total of 2 mg of iron/kg/day as ferrous sulfate starting at 2 weeks of age. Source: From Lundström et al. (1977).
Childhood and Adolescence
After infancy, iron deficiency becomes less common (Yip et al., 1987a,b) as the rate of growth decreases and the diet becomes more diversified. During adolescence, however, the prevalence of iron deficiency rises again (LSRO, 1984) because iron needs increase with the adolescent growth spun (Dallman, 1992) (Figure B-12). Presumably, iron deficiency is even more common among pregnant adolescents, in whom the iron needs for pregnancy follow closely after the increased needs for growth. However, no iron deficiency prevalence data for pregnant adolescents in the general population are available.

Figure B-12 Iron needs of male and female adolescents. There is an increase in the need for absorbed iron with the adolescent growth spurt, helping to account for an increased prevalence of iron deficiency at this age. The top of the figure is based on a representative male whose peak adolescent growth is at 13.5 years, near the average in the United States. Iron needs peak sharply during the growth spurt but decline rapidly thereafter. Sexual maturation (progression from Tanner stages 2 to 5) begins about 2 years before peak growth; this signals the period of greatest iron needs. The bottom of the figure is based on a representative female whose peak growth is at 11.5 years, near the average in the United States. Although iron needs rise to a maximum during peak growth, they remain high subsequently in females, since the iron needs to replace menstrual iron loss begin about I year after peak growth. Source: Dallman (1992).
Absorption of Iron from Food
The form of iron in the diet is even more important than the amount (Bothwell et al., 1989; Charlton and Bothwell, 1983; Cook et al., 1991; Hallberg, 1982; Hallberg and Rossander, 1952). Heme iron is better absorbed than non-heme iron, but non-heme iron makes up about 90 percent of the iron in the diet, and its absorption is strongly influenced by enhancers and inhibitors of iron absorption consumed in the same meal. These influences are greatest among individuals whose storage iron is depleted
(Figure B-13) (Cook, 1990). The diets of most people in the United States are relatively rich in the two most important enhancers, meat and ascorbic acid, and are therefore quite good sources of absorbable or bioavailable iron (LSRO, 1989) (Figure B-14). Phytates and polyphenols are important inhibitors. Protein, per se, has a highly variable influence on iron absorption, ranging from the facilitating effect of animal tissue protein (meat) to the strong inhibitory properties of isolated soy protein.
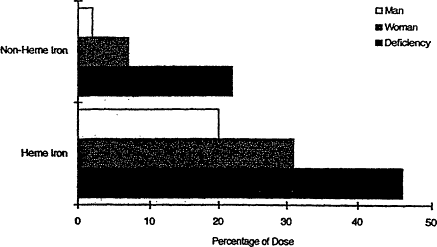
Figure B-13 Variation in absorption of heme and non-heme iron from a hamburger meal at different levels of iron status. Percent absorption from each form of iron is shown for an iron-replete man, a premenopausal woman, and a person with iron deficiency, individuals with assumed serum ferritin concentrations of 100, 30, and 10 µg/liter, respectively. Source: From Cook (1990).
The beverage consumed with a continental breakfast has a major impact on the amount of iron absorbed from the entire meal (Figure B-15). Tea and coffee inhibit iron absorption when consumed with a meal, whereas orange juice and other sources of vitamin C (ascorbic acid) are major enhancers. There is less influence on iron absorption when these beverages are consumed between meals. Meals that contain inhibitors of iron absorption, such as polyphenols (tannins in tea and certain vegetables) or phytate (in whole-grain cereals), can nevertheless become good sources of absorbable iron if they also include ascorbic acid as an enhancer (Siegenberg et al., 1991). Along similar lines, Galan et al. (1991) found that, contrary to expectations, good calcium sources such as low-fat milk or yogurt did not significantly depress iron absorption when consumed with a meat-containing meal. Thus, nutritionally valuable foods that happen to inhibit iron absorption need not be avoided if the diet contains adequate enhancers of iron absorption.

Figure B-14 Iron absorption as the percentage of iron absorbed from a diet varies according to the amount of the important enhancers of iron in the diet. The following estimates are based on the percent absorption of non-heme iron by individuals with no body iron stores from three different types of diets. A daily diet with low iron bioavailability is one containing less than 30 g of meat, poultry, or fish (lean, raw weight) or less than 25 mg of ascorbic acid. The comparable figures for a diet with medium iron bioavailability are 30 to 90 g of meat, poultry, or fish or 25 to 75 mg of ascorbic acid. A diet with high iron bioavailability is one containing more than 90 g of meat, poultry, or fish or more than 75 mg of ascorbic acid. Alternatively, it is one containing 30 to 90 g of meat, poultry, or fish plus 25 to 75 mg of ascorbic acid. Most diets in the United States are of medium to high iron bioavailability. Source: Data from Monsen and coworkers (1978), in Bothwell and Charlton (1981).

Figure B-15 Effect of beverage type on iron absorption. The beverage consumed with a meal (a continental breakfast, for example) has a large effect on the amount of iron absorbed from the entire meal. Source: Data from Rossander et al. (1979), in Bothwell and Charlton (1981).
Cook and colleagues (1991) recently obtained results indicating that the magnitude of differences in non-heme iron bioavailability might be less than absorption studies of single meals would suggest, particularly among those who are not iron deficient. When non-heme iron absorption was measured from the whole diet over a 2-week period, the difference between an iron absorption-enhancing and an iron absorption-inhibitory diet was 2.5-fold. In contrast, data based on a single meal showed a much larger difference (5.9-fold). Similar questions were raised by an earlier study of long-term administration of ascorbic acid, which might have been expected to increase iron stores, but it appeared to have little or no effect on serum ferritin levels (Cook et al., 1984). It was concluded that in the context of the U.S. diet, the role of enhancers was less important than the role of inhibitors of non-heme iron absorption (Cook et al., 1991). This topic deserves further investigation, since it is highly relevant to providing advice on how to improve iron nutrition. More information is needed regarding the effectiveness of dietary intervention (decreasing the consumption of inhibitors and increasing that of enhancers of iron absorption in the diet in the treatment or prevention of iron deficiency anemia).
For adults and children 2 years of age and older, the guidelines in Nutrition and Your Health: Dietary Guidelines for Americans (DHHS/USDA, 1991) at present provide a basis for good general nutrition and iron nutrition. In addition, for enhancement of iron absorption from an entire meal in individuals at risk of iron deficiency, it is advisable to include a good source of ascorbic acid or meat, fish, or poultry as part of the meal. Beverages, like tea and coffee, that inhibit iron absorption are best consumed between meals.
Absorption of Iron from Iron-Fortified Foods
Absorption of iron from iron-fortified foods has been a major factor in the declining prevalence of iron deficiency anemia among infants and children (Bothwell and MacPhail, 1992; Cook and Bothwell, 1984; Hurrell, 1992). Even the use of fortified cereal products, however, cannot be expected to prevent iron deficiency anemia among women of childbearing age who have unusually high menstrual blood losses (Swiss and Beaton, 1974). The impact of the iron fortification of cereal products on the iron nutrition of other women is unknown, but it may help to account for the relatively low prevalence of iron deficiency anemia, about 3 percent among nonpregnant white women, in the United States (LSRO, 1984).
Ferrous sulfate is commonly used to fortify infant formula and other products sold in cans and other airtight containers. Ferrous sulfate is also used to fortify bread and other bakery products that have a short shelf life. Since ferrous sulfate is highly soluble, it is as well absorbed as the intrinsic iron in these foods. However, compounds such as ferrous sulfate are not suitable for fortifying many foods that are marketed and stored for long periods in air-permeable packages, because most highly soluble forms of iron promote fat oxidation and rancidity. For this reason, elemental iron powders are commonly used to fortify such foods. The elemental iron powder used to fortify infant cereal contributes significantly to the prevention of iron deficiency anemia (Walter et al., 1993).
In Europe, the relatively nonreactive and insoluble ferric orthophosphate and ferric pyrophosphate are also widely used. There is less quantitative information about the effectiveness of these less well absorbed forms of iron used to fortify foods.
Absorption of Iron from Iron-Containing Supplements
Absorption of iron from iron-containing supplements is influenced by the dose, the iron stores of the recipient, whether iron is taken with or between meals, and whether it is taken alone or as part of a vitamin-mineral supplement (IOM, 1990a). The percentage of iron absorbed is high at the lowest doses and decreases substantially as the dose is increased. This is an important factor for clinicians to bear in mind, particularly in the treatment of iron deficiency anemia, because compliance is likely to be impaired by the substantial prevalence of gastrointestinal side effects when doses are increased to greater than 120 mg/day. In general, iron absorption from supplements is greatest in iron deficient individuals, because as mentioned above in respect to food iron, absorption is inversely proportional to iron stores.
Iron supplements are absorbed about twice as well when given between meals rather than with meals. It is also better to give an iron supplement with water or juice than with a beverage that is known to inhibit iron absorption, such as tea, coffee, or milk.
Slow-release iron supplements of various kinds have been developed to decrease the prevalence of side effects when large doses are used. These preparations are typically more expensive than commonly used, rapidly soluble forms of iron, such as ferrous sulfate, ferrous gluconate, and ferrous fumarate (Kastrup, 1992). When given with a meal, slow-release preparations may be better absorbed than ferrous sulfate, but they are less well absorbed under fasting conditions (Ekenved et al., 1976).
Women in their childbearing years commonly take iron as part of a vitamin-mineral tablet. Calcium and magnesium are the constituents of such tablets that are most likely to inhibit iron absorption (Babior et al., 1985; Seligman et al., 1983).
Definitions of Anemia, Iron Deficiency Anemia, and Iron Deficiency
Anemia
Anemia is defined as a hemoglobin concentration (or hematocrit) that is below the 95 percent range for healthy, well-nourished individuals of the same age, sex, and stage of pregnancy. Hemoglobin values are normally lower in children than in nonpregnant adults (Table B-3). During puberty, hemoglobin concentrations in males rise above those in females. During pregnancy, hemoglobin values gradually fall to a low point in the second trimester (Table B-4,
TABLE B-3 Hemoglobin and Hematocrit Cutoffs for Children, Nonpregnant Women, and Mena

Figure B-16 Hemoglobin values during pregnancy. The hemoglobin concentration normally declines during the fast half of pregnancy and rises during the second half. Values from 12 to 40 weeks of gestation are based on data from Svanberg et al. (1976), Sjöstedt et al. (1977), Puolakka et al. (1980), and Taylor et al. (1982). The baseline values (zero weeks) are based on LSRO (1984), and the 4- and g-week values are extrapolated from all these data and from Clapp et al. (1988). Source: CDC (1989).
TABLE B-4 Pregnancy Week-Specific Hemoglobin Cutoffs
|
|
Cutoff by Week of Gestationa |
|||||||
|
Parameter |
12 |
16 |
20 |
24 |
28 |
32 |
36 |
40 |
|
Mean hemoglobin, g/dl |
12.2 |
11.8 |
11.6 |
11.6 |
11.8 |
12.1 |
12.5 |
12.9 |
|
Fifth percentile hemoglobin values, g/dl |
11.0 |
10.6 |
10.5 |
10.5 |
10.7 |
11.0 |
11.4 |
11.9 |
|
Equivalent fifth percentile hematocrit values, % |
33.0 |
32.0 |
32.0 |
32.0 |
32.0 |
33.0 |
34.0 |
36.0 |
|
a For the sake of simplicity, hemoglobin cutoffs by trimester can be used as follows: 11.0 g/dl for the fast and third trimesters, based on 12 and 32 weeks of gestation, respectively, and 10.5 g/dl, based on 24 weeks of gestation for the second trimester. SOURCE: Based on pooled data from four European surveys of healthy women taking iron supplements (from CDC, 1989). |
||||||||
Figure B-16), largely because of a normal expansion in blood volume. From the end of the second trimester to term, the concentration of hemoglobin rises again.
Anemia is present only in those with hemoglobin concentrations that fall below the normal reference ranges for age, sex, and stage of pregnancy. Although iron deficiency is the most common cause of anemia, other causes include infection, hemoglobinopathies, and many other conditions. Although iron deficiency can result in anemia, high or excessive levels of iron intake do not increase the hemoglobin concentration beyond the normal range.
Iron Deficiency Anemia
Iron deficiency anemia
refers to an anemia that is associated with additional laboratory evidence of iron depletion, such as a low serum ferritin concentration, transferrin saturation, or mean corpuscular volume (MCV) or an elevation in erythrocyte protoporphyrin or transferrin receptor levels. Table B-5 lists the cutoff values for these tests in children and adults.
TABLE B-5 Cutoff Values for Tests of Iron Status
|
Age, year |
Serum Ferritin, µg/lite |
Transferrin Saturation, % |
Erythrocyte Protoporphyrin, µg/dl of red blood cells |
MCV, fl |
|
1-2 |
<10 |
<12 |
>80 |
<73 |
|
3-4 |
<10 |
<14 |
>75 |
<75 |
|
5-10 |
<10 |
<15 |
>70 |
<76 |
|
11-14 |
<10 |
<16 |
>70 |
<78 |
|
15-74 |
<12 |
<16 |
>70 |
<80 |
|
SOURCE: From LSRO (1984). |
||||
Serum ferritin concentration determination is the only laboratory test that allows the evaluation of iron reserves. A serum ferritin concentration of less than 10 µg/liter in children and less than 12 µg/liter in adults by itself indicates depleted iron stores (Table B-5). If an individual has already been found to be anemic, the likelihood of iron deficiency is greater than that in the general population. Under such circumstances, it may be appropriate to relax the cutoff value for the serum ferritin concentration. In combination with anemia, a value of less than 15 µg/liter indicates iron deficiency anemia.
Erythrocyte protoporphyrin accumulates in red blood cells when insufficient iron is available to form heme, the iron-containing portion of hemoglobin. It is most commonly measured on whole blood by a direct-readout instrument known as a hematofluorometer. Cutoff values are given in Table B-3. Erythrocyte protoporphyrin levels are elevated in individuals with iron defi-
ciency or lead poisoning, as well as in those with infections or inflammatory conditions of more than 1 week in duration. In an otherwise healthy individual, anemia accompanied by an elevated protoporphyrin level is most commonly indicative of iron deficiency anemia.
Other laboratory tests used in the diagnosis of iron deficiency anemia include MCV, serum iron concentration and iron-binding capacity, and transfer-tin receptor concentration. MCV is one of the red blood cell indices that is provided by many laboratories when a hemoglobin concentration is ordered. A low MCV is most commonly associated with iron deficiency or thalassemia trait.
The ratio of serum iron to iron-binding capacity, expressed as a percentage (transferrin saturation), is decreased in individuals with iron deficiency. It is used less frequently than in the past because its colorimetric analysis requires freshly separated plasma and its reproducibility is relatively poor because of large biologic variations.
The determination of transferrin receptor concentration is a promising new test that should shortly become available for widespread use. The transferrin receptor concentration is elevated in individuals with iron deficiency but not in those with inflammatory disease, a useful feature, particularly when both conditions coexist (Ferguson et al., 1992). For nutritional survey purposes, the combination of transferrin receptor, serum ferritin, and hemoglobin concentrations is likely to provide an excellent depiction of iron status (Cook et al., 1993).
Iron Deficiency
The term iron deficiency can be applied to a lack of iron that is severe enough to impair the production of red blood cells but not necessarily to the extent that the hemoglobin concentration falls below the normal reference range. Iron deficiency can progress to iron deficiency anemia.
Iron Deficiency Without Anemia
Iron deficiency without anemia represents a relatively mild iron deficiency that is diagnosed on the basis of a combination of biochemical indicators of iron status but in which the hemoglobin concentration remains within the reference range. Unfortunately, no single indicator of iron status is diagnostic of iron deficiency. Cook et al. (1976) found that the prevalence of anemia among individuals with only one abnormal index of iron metabolism (low serum ferritin concentration, low serum iron concentration, low iron-binding capacity, or elevated erythrocyte protoporphyrin levels) was 11 percent, only slightly higher than the 8 percent in the entire population. In contrast, anemia was found in 28 percent of individuals with two abnormal values and 63 percent of those with
three abnormal values. This finding and the analysis of data from the second National Health and Nutrition Examination Survey (NHANES II) suggested that for survey purposes, two or three abnormal biochemical indicators of iron status were more indicative of an iron deficiency of biologically significant severity than was a single indicator (LSRO, 1984). The term impaired iron status was applied to an abnormality in two or three of three biochemical tests.
Combinations of tests that have been used in large surveys include erythrocyte protoporphyrin and transferrin saturation determinations with either serum ferritin concentration or MCV determination (LSRO, 1984). The cost of doing multiple tests and the complexity of interpreting results for individuals make it difficult to detect this stage of iron deficiency except in nutrition surveys.
Rationale for Detecting Iron Deficiency by Screening for Anemia
Hemoglobin and hematocrit are most commonly used to screen for iron deficiency because they are easily analyzed and reflect the largest iron compartment in the body. Furthermore, physiologic impairment is associated almost entirely with iron deficiency anemia. However, many individuals with milder degrees of iron deficiency are missed by screening for anemia because of the overlap in values between normal and iron deficient individuals. Hemoglobin and hematocrit values also vary with age, sex, and stage of pregnancy. This makes it important that clinicians use the appropriate cutoff values.
Use of a Second Laboratory Test
One approach to alleviating the problem of an overlap in hemoglobin and hematocrit values between normal and iron deficient individuals is to use a second test to help ensure that individuals are accurately categorized and treated. If such tests are to be used in large populations, they must be relatively inexpensive and should preferably provide a result rapidly enough to allow initiation of treatment on the same visit. Tests that are widely used for this purpose are determination of erythrocyte protoporphyrin and serum ferritin concentrations. Transferrin receptor is the newest measure of iron status. Both transferrin receptor and erythrocyte protoporphyrin concentrations become elevated when there is a rate-limiting supply of iron to combine with erythrocyte protoporphyrin to form heme and eventually hemoglobin. They are therefore early indicators of physiologically significant iron deficiency. Studies to date indicate that the determination of transferrin receptor concentration will be particularly useful in the diagnosis of iron deficiency during pregnancy, because, like erythrocyte protoporphyrin, levels in iron supplemented individuals remain stable throughout gestation (Carriaga et al., 1991; Cook et al., 1993), in contrast to the other measures of iron status.
Erythrocyte protoporphyrin levels are elevated in individuals with iron deficiency and lead poisoning, and are therefore used to screen infants and young children for both, particularly those who live in low-income urban areas, where the two conditions are most common. Test results can be obtained rapidly and at low cost. This laboratory measure has not been widely used to test pregnant subjects, but it deserves further study because it seems very promising (Schifman et al., 1987). Erythrocyte protoporphyrin levels remain stable throughout pregnancy in iron supplemented women, but the levels rise in at least some unsupplemented women (Romslo et al., 1983).
Serum ferritin concentration is commonly determined in women, especially during pregnancy (IOM, 1990a). The serum ferritin concentration declines as iron stores decline. It is therefore valuable in predicting when iron deficiency is likely to develop during pregnancy in nonanemic women. Indeed, some advocate that pregnant women routinely be screened by determining their hemoglobin and serum ferritin concentrations (Hibbard, 1988). This option allows deferral of iron administration when both laboratory tests are normal. The results of Taylor et al. (1982) suggested that the serum ferritin concentration determination is less useful for this purpose in the last half of pregnancy because values decline to about 15 µg/liter even in iron supplemented women. Less marked declines were also noted by Romslo et al. (1983). However, Puolakka et al. (1980) and Wallenburg and van Eijk (1984) found that mean serum ferritin concentrations did not fall below 45 and 47 µg/liter, respectively, in iron supplemented women, compared with lows of 21 and 19 µg/liter, respectively, in the unsupplemented groups. There thus remains some degree of uncertainty about the usefulness of serum ferritin concentration determination in distinguishing iron supplemented from unsupplemented and potentially iron deficient individuals in the last half of pregnancy.
In nonpregnant women and children, a serum ferritin concentration of less than 12-15 µg/liter indicates that iron stores are very low, and a value in this range in combination with anemia provides strong evidence that the anemia is due to iron deficiency. Analysis of the serum ferritin concentration may be particularly useful for confirming that an anemia is due to iron deficiency, especially if it persists after iron treatment. This is especially applicable to blacks, among whom a slight depression in hemoglobin concentration may be normal (see below). Serum ferritin concentration analysis is also helpful for determining whether supplemental iron is necessary when it is poorly tolerated, despite adjustments in the iron supplementation regimen. If there is no anemia and if the serum ferritin concentration is not depressed, it is safe to delay iron supplementation until repeat analyses of serum ferritin indicate that iron stores have become very low. At present, the major disadvantage of the serum ferritin analysis is the delay in obtaining the results.
When routine screening is recommended for detecting iron deficiency among infants and women during their childbearing years, it is not cost-effective to use more than two or at the most three laboratory tests to distinguish iron deficient from nondeficient individuals. The high cost of laboratory stud-
ies, the relatively poor sensitivity and specificity of hemoglobin and other iron status measures (Dallman and Reeves, 1984), and the rapid change from iron adequacy to iron deficiency over a few months argue against using a larger battery of tests.
Consequences of Iron Deficiency
Consequences of Iron Deficiency During Infancy and Childhood
Iron deficiency anemia can impair energy metabolism, temperature regulation, immune function, and work performance (Dallman, 1986). However, the consequence of greatest concern in infants is the impairment of mental and psychomotor development that is associated with even mild iron deficiency anemia. The studies of Walter et al. (1989) (Figure B-17), Lozoff et al. (1991), and Idjradinata and Pollitt (1993) indicate that significant deficits occur at 12 months of age with a hemoglobin concentration of less than 10.0 g/dl and may be present when the hemoglobin concentration is between 10.0 and 10.9 g/dl (<11.0 g/dl is the lower limit of the normal 95 percent reference range at this age). Iron treatment reversed the anemia, but in two of the studies (Lozoff et al., 1991; Walter et al., 1989), it was found that impaired cognitive function may remain evident 4 to 5 years later when the formerly anemic infants enter school. A different conclusion regarding the reversibility of mental and psychomotor delay in infants with iron deficiency anemia emerged from the recent study of Idjradinata and Pollitt (1993), in which a double-blind, iron versus placebo treatment trial provided strong evidence of complete recovery in response to iron treatment (Table B-6). The issue of reversibility remains the subject of intensive study. Earlier studies of Pollitt et al. (1989) also show that schoolchildren with iron deficiency anemia have poorer cognitive function, which is only partly improved by iron treatment. These results indicate that anticipation and prevention of iron deficiency anemia should have higher priorities than its detection. An emphasis on the detection of anemia alone may not ensure prevention of the harmful consequences of iron deficiency anemia.
Consequences of Iron Deficiency During Pregnancy
For many years, clinical studies of iron deficiency during pregnancy suggested that the fetus remained an effective parasite with respect to iron status, even though anemia developed in the mother (for reviews, see Hallberg, 1992, and IOM, 1990b). This conclusion was based on data showing that newborns of mothers with mild iron deficiency anemia at term were not anemic and had normal or only slightly diminished iron stores, as estimated on the basis of the serum ferritin concentration. During the past decade, this concept that the fetus is protected from maternal iron deficiency has been challenged by the observa-
tion that anemia, and more specifically, iron deficiency anemia, in early pregnancy is associated with prematurity and low-birth-weight in the newborn rather than with anemia, as one might have anticipated. In addition to concern about fetal development in relation to iron deficiency anemia, there are also probably disadvantages to the mother. Adults with iron deficiency anemia have increased lactic acid levels and tachycardia with exercise (see the review by Dallman, 1986). There is also a report indicating that adults with iron deficiency anemia have impaired attention spans and short-term memories (Groner et al., 1986).

Figure B-17 Developmental delay in infants with iron deficiency anemia Developmental indices (mean ± standard error of the mean) were lower in 12-month-old infants with iron deficiency anemia (hemoglobin concentration, <10.5 g/dl) than in nonanemic infants. Source: From Walter et al. (1989).
Anemia and the Outcome of Pregnancy
Large retrospective studies by Garn et al. (1981) and Murphy et al. (1986) showed that anemia early in pregnancy was associated with prematurity, low-birth-weight, and increased infant mortality. These findings were recently confirmed and expanded in a large survey by the Centers for Disease Control and Prevention (CDC) (Kim et al., 1992). Women who had hemoglobin concentrations of less than 10.0 g/dl in the first trimester had a significantly higher incidence of low-birth-weight babies (11 percent) compared with women with hemoglobin concentrations of 10.0 g/dl or greater (7 percent).
TABLE B-6 Developmental Delay in Infants with Iron Deficiency Anemia
|
Group and Time in Relation to Treatment |
Hemoglobin, g/liter |
Mental Score |
Motor Score |
|||
|
Iron |
Placebo |
Iron |
Placebo |
Iron |
Placebo |
|
|
Iron deficiency anemia |
|
|
|
|
|
|
|
Before |
96 |
98 |
89 |
92 |
86 |
92 |
|
After |
129a |
107 |
108a |
93 |
112a |
98 |
|
Iron deficiency, no anemia |
|
|
|
|
|
|
|
Before |
126 |
126 |
102 |
102 |
103 |
104 |
|
After |
135a |
123 |
108 |
109 |
108 |
107 |
|
Iron sufficient |
|
|
|
|
|
|
|
Before |
126 |
126 |
105 |
105 |
105 |
106 |
|
After |
131 |
131 |
109 |
107 |
109 |
106 |
|
NOTE: Three groups of 12- to 18-month-old infants were enrolled in a double-blind, randomized iron versus placebo treatment trial for 4 months. Group 1 (n = 50) consisted of infants with iron deficiency anemia (hemoglobin concentration, <10.5 g/dl; transferrin saturation, ≤10 percent; serum ferritin concentration, <10 μg/liter). Group 2 (n = 29) consisted of infants with nonanemic iron deficiency (hemoglobin concentration, ≥12.0 g/dl; transferrin saturation, ≤10 percent; serum ferritin concentration, ≥12.0 μg/liter). Group 3 (n = 47) consisted of infants with iron sufficiency (hemoglobin concentration, ≥12.0 g/dl; transferrin saturation, >10 percent; serum ferritin concentration, ≥12.0 μg/liter). Abnormalities were reversed after 4 months of treatment. a p<0.01. SOURCE: Idjradinata and Pollitt (1993). |
||||||
Iron Deficiency Anemia and the Outcome of Pregnancy
A recent study by Scholl and coworkers (1992) indicates that a poor birth outcome (low-birth-weight and prematurity) is linked more specifically to maternal iron deficiency anemia (anemia and a low serum ferritin concentration) early in pregnancy than to anemia per se (Table B-7). The statistical analysis adjusted for known confounders, including maternal age, parity, ethnicity, and stage of gestation at first blood draw. Thus, it seems likely that maternal iron deficiency anemia contributes to prematurity and low-birth-weight, which are the most common causes of infant morbidity and mortality. Iron deficiency anemia was also associated with poor maternal weight gain during gestation. These findings are in accord with those of another study suggesting a link between low serum ferritin concentration and preterm labor (Ulmer and Goepel, 1988).
What is still lacking is direct cause-and-effect evidence that prevention of iron deficiency anemia by iron supplementation decreases the risk of low-birth-weight and prematurity. Such a study, involving randomization of a large group to iron and placebo treatments, although highly desirable, may be difficult to mount in the United States, where iron supplementation is so widely recommended and practiced. There would also be ethical constraints in withholding iron treatment from individuals with anemia and low serum ferritin concentrations. Two expert committees of the Institute of Medicine (IOM, 1990a, 1992)
and one formed by the Life Sciences Research Office (LSRO, 1991) recently agreed on recommending routine low-dose iron (30 mg/day) for pregnant women on the basis of a careful evaluation of currently available evidence. As in the case of infants, the weight of evidence leads to the conclusion that anticipation and prevention of iron deficiency anemia deserve a high priority.
TABLE B-7 Associations of Anemia and Iron Deficiency Anemia with Inadequate Weight Gain and Pregnancy Outcome
|
|
Anemia |
|||
|
Outcome |
Total |
Iron Deficiency |
Causes Other Than Iron Deficiency |
No Anemia |
|
Low-birth-weight Unadjusted, % |
17.1 |
25.9 |
15.9 |
12.2 |
|
AORa |
1.55 |
3.10 |
1.34 |
1.00 |
|
95% Confidence interval |
0.96-2.51 |
1.16-4.39 |
0.80-2.22 |
— |
|
Preterm delivery Unadjusted, % |
26.2 |
44.4 |
23.5 |
18.4 |
|
AORa |
1.30 |
2.66 |
1.16 |
1.00 |
|
95% Confidence interval |
0.86-2.24 |
1.15-6.17 |
0.76-1.79 |
— |
|
Small for gestational age Unadjusted, % |
11.1 |
8.3 |
11.5 |
7.5 |
|
AORb |
1.66 |
1.24 |
1.67 |
1.00 |
|
95% Confidence interval |
0.90-3.04 |
0.29-6.94 |
0.90-3.41 |
— |
|
Inadequate weight gain Unadjusted, % |
31.0 |
40.0 |
29.9 |
24.6 |
|
AORc |
1.62 |
2.67 |
1.51 |
1.00 |
|
95% Confidence interval |
1.10-2.36 |
1.13-6.30 |
1.02-2.25 |
— |
|
a AOR, adjusted odds ratio. Adjusted for maternal age, parity, ethnicity, prior low-birth-weight or preterm delivery, bleeding at entry, gestation at initial blood draw (entry), number of cigarettes smoked per day, and prepregnancy body mass index b Adjusted for maternal age, parity, prior low-birth-weight delivery, bleeding at entry, gestation at initial blood draw (entry), number of cigarettes smoked per day, and prepregnancy body mass index. c Adjusted for maternal age, parity, ethnicity, bleeding at entry, gestation at initial blood draw (entry), and prepregnancy body mass index. SOURCE: From Scholl et al. (1992). |
||||
Emerging Issues And Future Directions
Issues Involving the Possibility of Iron Excess
Iron is a micro nutrient with a relatively narrow range of optimal levels of intake. Furthermore, individuals' iron requirements vary substantially by age and sex. These characteristics are relevant to the fortification of general-purpose foods with iron. Fortification of infant formula and cereal with iron has been a resounding success in reducing the prevalence of anemia in infants (Yip et al., 1987a,b), probably because these iron-fortified foods have been so well accepted by health professionals and parents.
Fortification of general-purpose cereal products for older children and adults has been more controversial (Crosby, 1986). Although children and women of childbearing age are the target population, men who are at little risk of iron deficiency actually consume the greatest quantities of the fortified products. The concern about iron excess pertains primarily to men and postmenopausal women and involves the following issues: disease caused by oxidative damage, increased risk of infection, and interaction of iron with other minerals. (See also Appendix C.)
Disease Caused by Oxidative Damage
Iron is a catalyst in free-radical reactions, such as lipid peroxidation, which can cause tissue damage. Iron-binding proteins in plasma and tissues offer protection against such damage by binding ionic iron, but there are reasons to suspect that this protection is incomplete (Cross et al., 1987; Weinberg, 1984). Specific conditions in which there may be an association of clinical disease with high iron stores include ischemic heart disease (Salonen et al., 1992). A high-iron diet is a disadvantage for individuals with hemochromatosis, who absorb excess iron, which increases the body's iron burden and is then believed to cause free-radical damage to tissues. There is also evidence that increased iron stores can be associated with an increased rate of growth of various forms of cancer (Weinberg, 1992).
Increased Risk of Infection
The iron-binding proteins transferrin and lactoferrin are believed to provide protection from infection by competitively binding the iron that invading bacteria require for their growth (Weinberg, 1984). This phenomenon has been called nutritional immunity and is the basis for concern that unneeded iron could predispose an individual to infection. There is a large body of experimental evidence that supports this concept. However, there is no convincing evi-
dence that currently recommended iron fortification or treatment practices increase the risk of infection (Dallman, 1987).
Interaction of Iron with Other Minerals
Large doses of supplemental iron given to women (mean, 261 mg/day) resulted in a modest decline in plasma zinc levels (Hambidge et al., 1987). Another study of the relationship between iron and zinc or iron and copper indicated no such interaction (Sheldon et al., 1985). It remains uncertain whether the lower doses of iron that are currently recommended would increase the risk of zinc or copper deficiency. This question is relevant to the Institute of Medicine (1992) recommendation that if a therapeutic dose of iron (60-120 mg/day) is given, a multivitamin-mineral tablet containing 15 mg of zinc and 2 mg of copper given at a different time of the day should also be recommended. This would require two or three tablets per day and would result in poorer compliance than that with a simpler regimen of one tablet per day.
Race and Differences in Hemoglobin Concentration
The mean concentration of hemoglobin among groups of healthy blacks is consistently lower than that among a comparable, healthy white population of the same age and sex. This difference remains even when the populations have been screened to exclude those with iron deficiency and hemoglobinopathy and when the data are adjusted for socioeconomic differences. Indeed, the difference in hemoglobin concentration is similar in men and women, even though iron deficiency is a rarity among male adults. In NHANES II, this difference was 0.4 g/dl in children 3 to 4 years of age and 0.8 g/dl among both men and women between the ages of 20 and 44 years (LSRO, 1984). The whole hemoglobin distribution curve for blacks is shifted to the left of that for whites (Yip, 1989) (Figure B-18). The difference in hemoglobin concentration might be related to a very high prevalence of an α thalassemia gene among blacks (Beutler, 1988), but this has not been verified. Individuals with a single α thalassemia gene, unlike those with sickle trait and β thalassemia, are not detectable by hemoglobin electrophoresis, but only by laborious and costly techniques that are not clinically available. There does not appear to be any difference in hemoglobin values between whites and other racial groups, including Hispanics (Looker et al., 1989), East Asians (Dallman et al., 1978), Japanese (Uchida et al., 1988), and American Indians (Yip et al., 1984).
In general, the same hemoglobin criteria have been used for all races when screening women and children for anemia. This raises the problem that the percentage of black women and children who are erroneously suspected of having iron deficiency anemia is high enough to pose practical difficulties

Figure B-18 Hemoglobin distributions among white and black women ages 18-44 years. The concentration of hemoglobin is lower among black women than among white women. The figure is based on data from NHANES I. Source: From Yip (1989).
(Kim et al., 1992; Perry et al., 1992) (Figure B-19). Because blacks are more likely to be classified as anemic, they are more often subjected to unnecessary and expensive workups for anemia, needless concern about the possibility of disease, and the suspicion of poor compliance when there is no rise in hemoglobin concentration with iron therapy. The prevalence of anemia is substantially higher among pregnant black women than among those of other races. The difference between black and white or Hispanic women increases from about 8 percent in the first trimester to 15 to 20 percent in the third trimester (Figure B-19). It is uncertain to what extent the higher prevalence of ''anemia'' among black women, particularly late in pregnancy, is due to iron deficiency anemia. However, there is good evidence from NHANES II (LSRO, 1984) that black women have lower hemoglobin levels than white women even when their iron statuses are similar (Table B-8).
The problem of the specific criteria that should be used to diagnose anemia in blacks poses important health policy issues. Use of the same hemoglobin concentration criterion for women and children of all races is the simplest approach and recognizes the fact that not all blacks have low hemoglobin concentrations. However, many normal black individuals are incorrectly suspected of having iron deficiency anemia. This requires an awareness that the failure of a mild "anemia" in a black woman or child to respond to iron treatment is likely to indicate a normally low hemoglobin concentration and not necessarily a failure to take iron or an unusual anemia that requires further workup. The use of separate hemoglobin criteria for blacks could be seen as racially stigmatizing, but the advantage of fewer false-positive diagnoses is a strong argument for
making an appropriate downward adjustment in hemoglobin and hematocrit cutoff values (Johnson-Spear and Yip, in press).
Absorption of Iron from Multivitamin-Mineral Supplements and Certain Foods
Less iron is absorbed from certain multivitamin-mineral supplements than from an equivalent amount of iron given alone. Calcium carbonate and magnesium oxide appear to be particularly inhibitory to iron absorption (Babior et al., 1985; Seligman et al., 1983). Seligman et al. (1983) found that iron absorption almost doubled when calcium as calcium carbonate was decreased from 350 to 250 mg and magnesium as magnesium oxide was decreased from 100 to 25 mg. The use of calcium supplements may also inhibit absorption of iron from foods under certain conditions (Hallberg et al., 1991). However, Galan et al. (1991) found no inhibition of iron absorption when calcium as milk or yogurt was added to a standard, meat-containing meal. These findings demonstrate the importance of additional research to determine the optimal ratios of nutrients in prenatal supplements and the relative merits of foods versus supplements as sources of certain nutrients. For the time being, if iron is given in the form of a prenatal supplement, it seems advisable to select products that contain about 60 mg of iron rather than use the dose of 30 mg that is recommended if iron is given alone. This would allow for an anticipated lower percentage of iron absorbed. It would also be best to select a formulation that contains no more than 250 mg of calcium.
Another area that needs further investigation is the availability of iron from certain foods, particularly breakfast cereals that contain iron and other micronutrients at the adult Recommended Dietary Allowance (RDA) equivalents. Specially formulated fortified foods targeted to women of childbearing age could be developed and might be of potential importance as vehicles for iron, folate, and possibly, calcium. With appropriate studies to verify the bioavailability of iron, such foods might be an attractive alternative to women for whom compliance in taking tablets is a problem.
Association of Neural Tube Defects with Folate Deficiency in Early Pregnancy: Implications for the Prevention of Iron Deficiency
The CDC recently recommended that all women of childbearing potential should consume 0.4 mg of folic acid per day for the purpose of reducing their risk of having a pregnancy affected with spina bifida or other neural tube defects (CDC, 1992). Currently in the United States, almost 2,500 infants are born with these defects each year. The recommendation was based largely on a recent investigation showing a reduced prevalence of first occurrence of neural tube defects in infants of women taking folate-containing supplements from
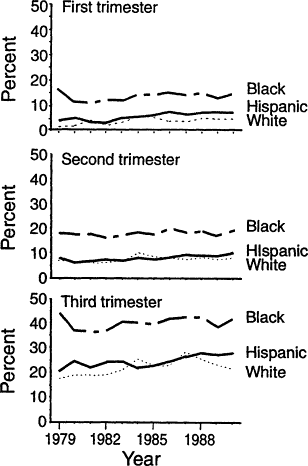
Figure B-19 Antepartum anemia, by racial or ethnic group and trimester, determined as pan of the Pregnancy Nutrition Surveillance System, United States, 1979-1990. The prevalence of anemia increases with the progression of pregnancy. There has been no decrease in the prevalence of anemia during the period monitored, a decade during which there was a marked diminution of anemia among infants and preschool children. The consistently higher prevalence of anemia among blacks than among Hispanics or whites (about 8 percent in the first trimester, 10 percent in the second trimester, and 15 to 20 percent in the third trimester) is partly due to the lower hemoglobin concentrations in blacks, even those with normal iron status. Source: From Kim et al. (1992).
TABLE B-8 Prevalence of Anemia and Iron Deficiency Among White and Black Women from 20 to 44 Years of Age
|
|
Percent |
|
|
Condition |
White |
Black |
|
Anemia |
9.0 |
25 |
|
Iron deficiency |
5.0 ± 0.6a |
5.7±0.9 |
|
NOTE: Anemia was defined as a hemoglobin concentration of <12.0 g/dl. Individuals were designated as having iron deficiency when results of two or three tests (MCV, transferrin saturation, and erythrocyte protoporphyrin) were abnormal. Although anemia was much more common among blacks than among whites, the prevalence of iron deficiency was similar. a Values are means ± standard errors of the means. SOURCE: LSRO (1984). |
||
at least 1 month before conception to the date of the second missed menstrual period or later (Czeizel and Dudas, 1992). Several earlier studies indicated that the prevalence of recurrent neural tube defects could be decreased by folate supplementation.
The relevance of the CDC recommendation to iron supplementation is that many more women of childbearing age will be taking folate in the form of a multivitamin-mineral preparation that also contains iron before they are pregnant and during early pregnancy. The recent studies associating iron deficiency anemia or anemia in early pregnancy with low-birth-weight will also foster increased and earlier use of iron supplements.
Between 1976 and 1980, 38 percent of women between the ages of 18 and 50 reported taking a vitamin supplement, a mineral supplement, or both (Laplan et al., 1986). In the more recent 1987 Health Interview Survey, 27 percent of women reported daily supplement use, and for 20 percent this was a multivitamin-mineral supplement (Subar and Block, 1990). The prospects of increasing the use of vitamin-mineral supplements as a vehicle for iron supplementation make it imperative that there be more investigation of the availability of iron from such preparations, as suggested above. Since folate-fortified foods are being considered as a means of supplying extra folate to women of childbearing age, there is also the need to investigate the prospects for developing special fortified foods targeted to young women. Such food could respond to concerns not only about folate deficiency but also about iron deficiency, osteoporosis, and other problems primarily involving this group.
Iron Supplements Often Are Not Taken Regularly
Recommendations to use iron supplements have little prospect for success unless they are likely to result in reasonably good compliance. Unfortunately, compliance can be surprisingly inconsistent even for relatively simple drug regimens used for life-threatening conditions like epilepsy, diabetes, hyperten-
sion, and organ transplantation (Haynes et al., 1979). The problem is likely to be even greater when the individual has no obvious illness. Bonnar et al. (1969) monitored compliance by measuring iron levels in the stools of pregnant women who were advised to take ferrous fumarate at a dose of 65 mg of iron twice a day. About one-third failed to take iron, and this proportion was even greater among those who were initially anemic. The poor compliance could not be attributed to gastrointestinal side effects, since these were extremely rare.
The side effects of iron therapy would contribute to poor compliance, but their prevalence has been most thoroughly investigated in double-blind studies with doses higher than those recommended in this report. At a dose of 65 mg of iron as ferrous sulfate taken three times a day, about 25 percent of subjects had side effects, whereas 13 percent of those receiving a placebo experienced side effects (Hallberg et al., 1967; Sölvell, 1970). With a doubling of the dose, the prevalence of side effects increased to 40 percent. There is little information about side effects at lower doses, but evidence of dose dependency and the study of Bonnar et al. (1969) cited above make it unlikely that doses of 30 mg of iron once a day or 60 mg twice a day would be a major deterrent to compliance. Nevertheless, it would be helpful to have more information on side effects at these lower doses.
Improving Compliance
What can be done to improve compliance? Compliance can be improved by using strong motivational techniques and frequent follow-up. Under ordinary circumstances, however, especially in busy and understaffed clinics, it seems likely that poor compliance is frequently responsible for a failure to treat or prevent iron deficiency anemia. The most effective single approach is to simplify the treatment regimen. Compliance was found to be substantially better with once-a-day iron therapy than with three-times-a-day medication (Porter, 1969) (Figure B-20). A similar result was obtained with long-term treatment for epilepsy (Cramer et al., 1989). Compliance averaged 87 percent with once-daily treatment, 81 percent with twice-daily treatment, 77 percent with three-times-daily treatment, and 39 percent with four-times-daily treatment. It would clearly be desirable to fill all supplement needs whenever possible with a single tablet taken once a day.
There are additional measures that are likely to be helpful in improving compliance (Haynes, 1979) (Table B-9). It is important that the health care provider explain why the medication will benefit the patient or her child. Medication is most likely to be taken if the health care provider provides written or printed instructions. Taking of medication should be linked to an already established daily routine like tooth brushing. On revisits to an office or clinic, it is important for the health care provider to reinforce the use of the supplement by asking about it and stressing its importance. Even so, compliance is likely to drop off between visits. A group of epileptic patients averaged 88 percent
compliance before and 86 percent after a clinic visit, but this dropped to 67 percent compliance a month later (Cramer et al., 1990).
Iron Deficiency Increases the Risk of Lead Toxicity
Iron deficiency is associated with increased levels of lead in the blood of preschool children (Clark et al., 1988). The basis for this association is probably the shared absorptive mechanism for the two metals. Lead absorption is increased in individuals with iron deficiency. Since lead toxicity impairs cognitive function (as does iron deficiency), this risk is another justification for preventing iron deficiency.
As mentioned above, iron-fortified foods have been remarkably effective in reducing the prevalence of iron deficiency anemia among infants and preschool children, thereby decreasing the vulnerability to lead poisoning. Environmental exposure to lead has decreased since leaded gasoline has been removed from the market (Annest et al., 1983); removal of additional lead from the environment will be much more costly. At present, prevention of iron deficiency anemia is probably the most cost-effective way of further decreasing the risk of lead poisoning.
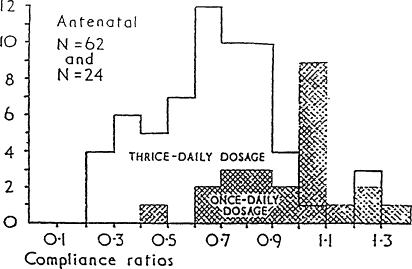
Figure B-20 Distribution of compliance ratios for antenatal patients on thrice- daily and once -daily iron preparations (compliance ratio is the number of tablets actually taken by the patient divided by the number of tablets that should have been taken). Source: Based on Porter (1969).
TABLE B-9 Methods for Improving Compliance
|
|
|
|
|
Iron Deficiency Anemia In Infants And Children: Prevalence And Recent Recommendations
Magnitude of the Problem
Iron deficiency is common among infants and children from about 6 months to 3 years of age, but its prevalence has been declining over the past two decades. The most recent information on the prevalence of iron deficiency in the U.S. population as a whole, which was collected between 1976 and 1980, is from NHANES II. NHANES III is now in progress and should soon provide more up-to-date information. In NHANES II, the prevalence of iron deficiency was about 9 percent in infants aged 1 to 2 years, which was determined by using the same laboratory criteria (on the basis of MCV, transferrin saturation, and erythrocyte protoporphyrin) that yielded a prevalence of 5 percent among women during their childbearing years. Since then, the prevalence of iron deficiency anemia in infants and preschool children has been declining drastically on the basis of the prevalence of anemia in clinics that participate in the U.S. Department of Agriculture's Supplemental Food Program for Women, Infants, and Children (WIC) (Yip et al., 1987b) (Figure B-21) and in private practice settings (Yip et al., 1987a) (Figure B-22). In WIC clinics, the prevalence of anemia declined in a linear fashion, from 7 percent in children born in 1973 to 3 percent in those born in 1984. Stricter criteria for anemia than those commonly used in a clinical setting (hemoglobin, <10.3 g/dl; hematocrit, <31 percent) were applied to evaluate trends more accurately because of evidence that reporting of the measurements was influenced by the enrollment requirements for WIC. The actual values are apt to underestimate the prevalence of anemia by more widely used clinical criteria (AAP, CON, 1993; Dallman and Siimes, 1979). In a middle-class population, the prevalence of anemia on the basis of the widely used cutoff of a hematocrit level of less than 33 percent between the ages of 9 and 23 months declined from 7 percent between 1969 and 1973 to less than 3 percent between 1982 and 1986 (Figure B-22). The latter is close to the statistical baseline level for a normal population, suggesting that there remains little or no iron deficiency anemia in this study population. These striking improvements can be attributed to changes in infant feeding practices that took place during this period: later introduction of cow's milk and greater use of iron-fortified formula (Fomon, 1987). The incorporation of iron-fortified for-
mula in the WIC food package for infants is believed to have played a major role in the decline of anemia among infants from lower socioeconomic groups.
In screening for anemia, mild infection has come to be recognized as an important confounding factor. Young children commonly have mild upper respiratory infections, gastroenteritis, or otitis media on health care visits. In one study, the prevalence of anemia between 1982 and 1986 in children with illnesses averaged about ? percent compared with 3 percent in those from the same population who were entirely well (Yip, 1989) (Figure B-23). Even a history of a recent infection or evidence of an inflammatory process (on the basis of erythrocyte sedimentation rate) is associated with a much higher prevalence of anemia and low-normal hemoglobin values (Figure B-24). These findings emphasize the importance of testing for anemia to screen for iron deficiency only when an individual is and has recently been free of infection.
Preterm Infants
Studies comparing the concentration of hemoglobin and other iron status measures in iron supplemented and unsupplemented infants show that those with a birth-weight of less than 2,000 g are at very high risk of developing iron deficiency anemia after 2 months of age unless they receive an iron supplement, iron-fortified formula, or both (Lundström et al., 1977) (Figure B-11).

Figure B-21 Prevalence of anemia among infants and children in WIC clinics. The lower prevalence in children already in the WIC program is associated with the use of iron-fortified infant formula and other foods intended to improve iron status. Source: Comparison of anemia trends of non-WIC and WIC children is based on data from the CDC Pediatric Nutrition Surveillance System, 1976-1985, from Yip et al. (1987b).
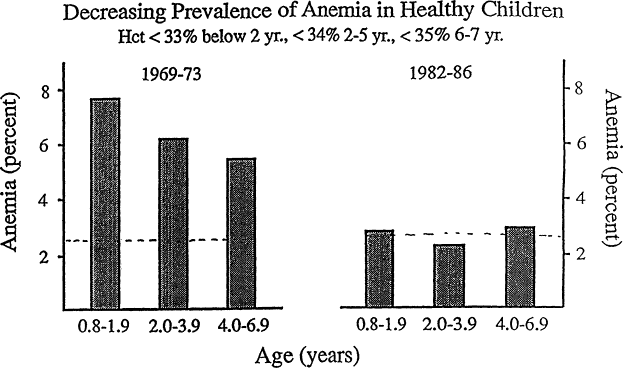
Figure B-22 Prevalence of anemia among a group of middle-class U.S. children from 0.8 to 6.9 years of age. Anemia has become rare among middle-class children. Between 1969 and 1981, there was a decline in the prevalence of anemia with increasing age. Between 1982 and 1986, the prevalence of anemia in all age ranges fell close to the theoretical minimum of 2.5 percent (horizontal dashed line). Hematocrit cutoff values for anemia are <33 percent for those younger than 2 years of age, <34 percent for those from 2 to 5 years of age, and <35 percent for those from 6 to 7 years of age. Source: From Yip et al. (1987a).

Figure B-23 Comparison of the prevalence of anemia among healthy children and those with a mild illness over four time periods on the basis of data from a middle-class pediatric practice. The prevalence of anemia in children with a mild infection is much higher than the prevalence of anemia in healthy children. Both have been declining over the past two decades. Source: Adapted from Yip et al. (1987a).
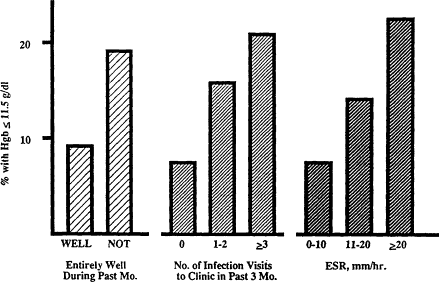
Figure B-24 Effect of recent infection or inflammatory process on anemia and hemoglobin value. Anemia and low to normal hemoglobin concentration are more common among healthy 1-year-old infants with a history of recent mild infection than among those who have been entirely well. ESR is erythrocyte sedimentation rate. Source: From Reeves et al. (1984).
Preschool Children, School-Age Children, And Adolescents
After age 3 years, the risk of iron deficiency anemia is less (Yip et al., 1987a,b) (Figure B-22). When children are served meals in day care or at schools, there is an opportunity to provide foods that meet nutrition guidelines for iron and other nutrients. In adolescence, it becomes more difficult to meet the increasing iron needs (Hallberg et al., in press; Widholm et al., 1967) because meals are often eaten with less regularity and snack foods may substitute for what was previously a more varied diet. It is also a challenge to screen teenagers for anemia because it is uncommon for them to be seen for annual health maintenance checkups as recently recommended by the American Medical Association Panel on Adolescent Health (AMA, 1992).
Review of Earlier Guidelines for Prevention and Treatment
Committee on Nutrition, American Academy of Pediatrics
The Committee on Nutrition (CON) of the American Academy of Pediatrics (AAP) has issued a number of statements on iron nutrition and iron metabolism since 1969. For the most part, the recommendations have remained the same. The changes during that period have involved mainly the duration for which iron-fortified formula was to be used and the age before which cow's milk should not be started in the diets of infants. The recommendations for term and preterm infants that apply currently can be summarized as follows.
Term Infants
On the basis of an extensive review of iron balance in infancy, the recommended total iron intake for term infants is 1 mg/kg/day to a maximum of 15 mg/kg/day (AAP, CON, 1969). All formula-fed infants should receive an iron-fortified formula (AAP, CON, 1989) until 12 months of age. Iron-fortified formula supplies ample iron to meet the iron requirements of infants. Breastfed infants should be weaned to an iron-fortified formula rather than to cow's milk if they are weaned before 12 months of age. Cow's milk and low-iron formulas should not be fed during the first 12 months of life (AAP, CON, 1992). Iron-fortified infant cereal is recommended when infants start solid foods (AAP, CON, 1969, 1976).
Preterm Infants
The recommended total iron intake for preterm infants is 2 to 3 mg/kg/day to a maximum of 15 mg/kg/day (AAP, CON, 1969, 1985). Iron supplementation for low-birth-weight infants should start when the infant reaches a body weight of about 2,000 g or goes home. Infants fed human milk should receive 2 to 3 mg of elemental iron (per kg/day) as ferrous sulfate drops. If oral iron supplements are started earlier, at 2 weeks or when enteral feedings are tolerated, a dose of 2 to 3 mg/kg/day as well as a vitamin E supplement should be given. Formulas with iron usually contain sufficient iron so that no additional iron supplement is needed. Breastfed infants should be weaned to an
iron-fortified formula rather than to cow's milk if they are weaned before 12 months of age. Cow's milk and low-iron formulas should not be fed during the first 12 months (AAP, CON, 1992). Iron-fortified infant cereal is recommended when infants start solid foods (AAP, CON, 1967, 1976).
Canadian Pediatric Society
Breastfed infants should be fed iron-fortified cereal (Canadian Pediatric Society, Nutrition Committee, 1991). Iron-fortified formula should be fed to infants who are not breastfed. Cow's milk should not be started until after 9 to 12 months of age. Iron supplements for low-birth-weight infants should start by 8 weeks of age and should be maintained until 12 months of age.
Recommended Dietary Allowances, 10th Edition
Infants to Age 3 Years
For infants from birth to age 3 years, 1 mg of iron per kg/day was recommended for those who are not breastfed, but it was noted that satisfactory levels of hemoglobin could be maintained without extra iron from birth to age 3 months. The RDA for iron for those aged 6 months to 3 years was set at 10 mg/day. For low-birth-weight infants with birth-weights of from 1,000 to 2,500 g, 2 mg/kg/day was recommended starting no later than 2 months of age. The maximum iron intake was set at 15 mg/day for term and low-birth-weight infants (NRC, 1989).
Children Ages 3 Years and Older
For children, the RDA for iron was set at 10 mg/day, with an increase at age 10 years to 12 mg/day for males and 15 mg/day for females (NRC, 1989).
Iron Deficiency In Women Of Childbearing Potential: Prevalence And Recent Recommendations
Magnitude of the Problem
In contrast to children in whom inadequate iron intake is usually responsible for iron deficiency, excessive iron losses via menstrual blood and pregnancy play major roles among women of childbearing age. Also in contrast to children, there is no evidence that the prevalence of iron deficiency among women of childbearing age has declined substantially during the past two decades.
Nhanes II (1976-1980)
In this large national survey, about 5-10 percent of women between the ages of 20 and 44 years in the United States were iron deficient on the basis of
two or three abnormal values for indicators of iron status (Expert Scientific Working Group, 1985; LSRO, 1984). Five percent were iron deficient on the basis of an abnormal MCV, transferrin saturation, and erythrocyte protoporphyrin values; and 10 percent were iron deficient on the basis of abnormal serum ferritin, transferrin saturation, and/or erythrocyte protoporphyrin values. In contrast, less than I percent of men between the ages of 20 and 44 years were iron deficient by these criteria.
Females between the ages of 15 and 19 years had a prevalence of iron deficiency similar to that for women aged 20 to 44 years. The prevalence of iron deficiency among women between 20 and 44 years of age was greater with poverty, brief education, and high parity (LSRO, 1984) (Table B-10). There is no information on the prevalence of iron deficiency among a national sample of pregnant women.
About 8 percent of white women were anemic (hemoglobin concentration, <12.0 g/dl), and about 3 percent had iron deficiency anemia (anemia plus two or three abnormal hemoglobin values). These analyses were reported for whites only because many more blacks would be identified as anemic, despite having normal iron status (as discussed above; see Table B-8).
Pregnancy Nutrition Surveillance System—United States, 1979-1990
This CDC Surveillance System monitored nutritional risk factors among low-income pregnant women participating in public health programs. A recent
TABLE B-10 Risk Factors for Iron Deficiency
|
Risk Factor |
Percent with Iron Deficiency |
|
Economic status |
|
|
Above poverty level |
5.1 ± 0.5* |
|
Below poverty level |
7.8 + 1.5* |
|
Education |
|
|
None/elementary school |
|
|
High school |
5.4 ± 0.6** |
|
College |
4.2 ± 0.6+ |
|
Parity |
|
|
0 |
3.1±0.5 |
|
1-2 |
3.8 ± 0.8 |
|
3-4 |
9.4±1.1++ |
|
>5 |
11.1 ± 2.1++ |
|
NOTE: Iron deficiency is defined as two or three abnormal values for the following tests: MCV, transferrin saturation, and erythrocyte protoporphyrin. Prevalence of iron deficiency is shown for women between 20 and 44 years of age. Significance: *, p < 0.10; **,p <0.05; +,p <0.01; ++, p < 0.005 (in both other groups). SOURCE: LSRO (1984). |
|
report based on this survey (Kim et al., 1992) showed a prevalence of anemia that was high and that increased during the course of pregnancy. In 1990, for example, about 5, 8, and 25 percent of white and Hispanic women were anemic in the first, second, and third trimesters, respectively, indicating a worsening of iron status during the progression of pregnancy as iron needs increase (Figure B-19). Most discouraging was the finding that the prevalence of anemia has remained stable or has increased slightly since 1979 (Figure B-19), a period during which there has been a marked decline in anemia among infants and preschool-age children served by the same clinics (Figure B-21). These findings indicate a need for new approaches to the prevention of anemia and iron deficiency among low-income, pregnant women.
Black women had a disproportionately high prevalence of anemia compared with white and Hispanic women. The discrepancy increases progressively from the first to the second and third trimesters. The high prevalence is partly related to the lower hemoglobin concentrations in blacks, even when iron status is normal. The extent to which iron deficiency also plays a role in the higher prevalence of anemia among blacks, particularly in the second and third trimesters, remains uncertain. The findings do show that the interpretation of anemia among pregnant black women requires special attention to minimize the false diagnosis of anemia and iron deficiency.
Clinical Trials in Iron Supplemented Versus Unsupplemented Pregnant Women
Several carefully performed studies in which iron supplemented and unsupplemented women were followed at monthly intervals during pregnancy were recently summarized (Table B-4). In all cases, the women studied were not anemic on their first prenatal visit. However, the mean hemoglobin concentration became lower in unsupplemented than in iron supplemented women after about 24 weeks of gestation, and the difference, as illustrated by one of these studies (Taylor et al., 1982), continued to increase with the progression of pregnancy (Figure B-25). The increasing prevalence of anemia during the course of pregnancy is in accord with the survey data described above for the Pregnancy Nutrition Surveillance System.
Review of Current Guidelines
Committee on Professional Standards, American College of Obstetricians and Gynecologists
As early in pregnancy as possible, the clinician should determine the hemoglobin concentration or hematocrit (a venipuncture will be required for the additional laboratory tests that are recommended). The hemoglobin or hematocrit analysis should be repeated again early in the third trimester (ACOG, 1989).

Figure B-25 Hemoglobin concentration during gestation and postpartum in women receiving an iron supplement and women not supplemented with iron. The unsupplemented group had a lower mean hemoglobin concentration after about 24 weeks of gestation, and differences became more marked during the progression of pregnancy. After delivery, differences in hemoglobin concentration disappear as the blood volume decreases. Source: Data from Taylor et al. (1992).
Nutritional status should be evaluated on the initial visit and should be monitored on subsequent visits throughout the pregnancy. If iron, folate, and certain other vitamin needs are not met by the diet, the clinician should recommend a vitamin-mineral supplement that supplies the RDAs for pregnant women.
The recommended frequency of visits is every 4 weeks for the first 28 weeks of gestation, every 2 to 3 weeks until 36 weeks of gestation, and weekly thereafter.
Institute of Medicine: Nutrition During Pregnancy and Nutrition During Pregnancy and Lactation: An Implementation Guide
Nutrition During Pregnancy
(IOM, 1990a) included a chapter on iron nutrition during pregnancy with a review of the literature (IOM, 1990b). That review concluded with the recommendations outlined below. The Implementation Guide, which was published 2 years later (IOM, 1992), was prepared as a briefer user manual for improving nutrition during pregnancy and lactation. It included more specific clinical information, which is also summarized below.
Eat a well-balanced diet (Dietary Guidelines for Americans, DHHS/USDA, 1991) that contains enhancers of iron absorption (ascorbic acid, meat).
Routinely determine the hemoglobin concentration or hematocrit on the first prenatal visit to detect preexisting anemia. A hemoglobin level of less than 11.0 g/dl during the first or third trimesters or less than 10.5 g/dl during the second trimester is defined as anemia.
Nonanemic women should routinely take about 30 mg of ferrous iron beginning at about the 12th week of gestation. Take the iron between meals and with water or juice, but not with milk, tea, or coffee. The rationale for the 30-mg dose is that it is the lowest for which there is strong evidence of effectiveness (Chanarin and Rothman, 1971) (Figure B-26). Such low doses can be relatively effective because the percentage of iron absorbed decreases as the dose increases. Thus, a more than six fold increase in dose from 18 to 120 mg scarcely doubles the amount of iron absorbed (B-Figure 27) (Hahn et al., 1951). It was considered equally important to select a low dose to avoid the relatively high prevalence of side effects seen with the commonly employed regimens of 180 rag/day. The lower dose could be given as a single tablet once a day, favoring compliance.
Anemia accompanied by a serum ferritin concentration of less than 12 µg/liter can be presumed to be iron deficiency anemia and requires treatment with 60 to 120 mg of ferrous iron daily (no more than about 60 mg per dose). Hemoglobin or hematocrit should be checked again after about I month. When the hemoglobin or hematocrit becomes normal for the stage of gestation, the dose can be decreased to 30 mg/day. A persistent anemia requires questions about compliance or further laboratory studies to consider other causes of anemia.
Nutrition During Pregnancy and Lactation: An Implementation Guide (IOM, 1992)
A preconception or interconception visit should include a screening for anemia with the determination of hemoglobin concentration or hematocrit. A hemoglobin concentration of less than 12.0 g/dl was defined as anemia (Table B-4). Corrections for smoking and residence at high altitude were provided (Table B-11). The determination of serum ferritin should be considered in women with anemia. A serum ferritin concentration of less than 20 µg/liter with anemia indicates iron deficiency as the cause of anemia.
The use of a folate-containing supplement to help prevent recurrent neural tube defects was mentioned as relevant to iron supplementation, since it offers the possibility of using a vitamin-mineral supplement containing both iron and folate.
Prenatal multivitamin-mineral supplements were recommended for women identified to be at high nutritional risk. Table B-12 shows the suggested composition of such supplements.
A brief dietary history was recommended on this and the first prenatal visit. Dietary advice based on Dietary Guidelines for Americans was also suggested for both visits (DHHS/USDA, 1991).
The first prenatal visit includes screening for anemia. Anemia was defined as a hemoglobin concentration of less than 11.0 g/dl in the first or third
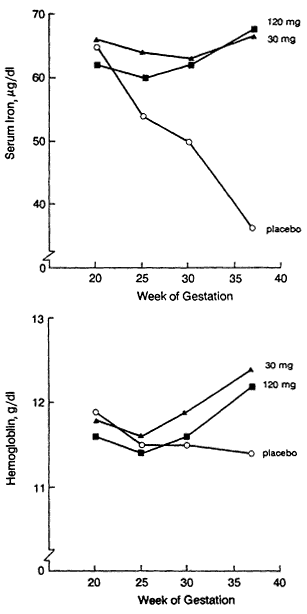
Figure B-26 Serum iron and hemoglobin levels in groups of 46 to 49 randomly assigned women receiving either a placebo or various doses of elemental iron as ferrous fumarate given orally on a daily basis. Data from groups receiving 60-mg oral doses of iron or 1 g of iron given parenterally and then 60-mg oral doses of iron per day are not shown but are similar to those of the iron supplemented groups shown in the figure. Note that daily doses of 30 and 120 mg were similarly effective in preventing anemia. Source: From IOM (1990a), based on Chanarin and Rothman (1971).

Figure B-27 Absorption of iron (as a percentage of the dose above and in milligrams below) in relation to dose and stage of pregnancy, based on data from Hahn et al. (1951). Iron absorption increased during the progression of pregnancy. At any stage of pregnancy, the percentage of iron absorbed decreases as the dose of iron increases. At about 30 weeks of gestation, a more than six fold increase in dose from 18 to 120 mg of iron only doubles the amount of iron absorbed. Source: IOM (1990a).
TABLE B-11 Cutoff Values for Anemia in Women of Childbearing Age
|
Group |
Hemoglobin (g/dl)a |
Hematocrit(%)a |
|
Nonpregnant women |
|
|
|
Nonsmokers |
12.0 |
36 |
|
10-20 cigarettes/day |
12.3 |
37 |
|
20-40 cigarettes/day |
12.5 |
37.5 |
|
Pregnant women |
|
|
|
Nonsmokers |
|
|
|
First trimester |
11.0 |
33 |
|
Second trimester |
10.5 |
32 |
|
Third trimester |
11.0 |
33 |
|
10-20 cigarettes/day |
|
|
|
First trimester |
11.3 |
34 |
|
Second trimester |
10.8 |
33 |
|
Third trimester |
11.3 |
34 |
|
21-40 cigarettes/day |
|
|
|
First trimester |
11.5 |
34.5 |
|
Second trimester |
11.0 |
33.5 |
|
Third trimester |
11.5 |
34.5 |
|
a For altitudes of about 5,000 ft. (i.e., Denver, Salt Lake City), add 0.5 g/dl to the hemoglobin cutoff or 1.5 percent to the hematocrit cutoff. SOURCES: IOM (1992) and CDC (1989). |
||
TABLE B-12 Suggested Composition (approximate) of Prenatal Daily Vitamin-Mineral Supplements for Use by Women Identified To Be At High Nutritional Riska
|
Nutrient |
Amount |
|
Iron |
30-60 mg |
|
Zinc |
15 mg |
|
Copper |
2 mg |
|
Calciumb |
250 mg |
|
Vitamin D |
10 µg (400 IU) |
|
Vitamin C |
50 mg |
|
Vitamin B6 |
2 mg |
|
Folate |
300 µg |
|
Vitamin B12 |
2 µg |
|
a If vitamin A is included, β-arotene is preferred over retinol, to reduce the risk of toxicity or other adverse reactions. b Since calcium and magnesium may interfere with iron absorption, upper limits of 250 and 23 mg per dose, respectively, are recommended as a part of vitamin-mineral supplements. Some calcium supplements provide less than the recommended 600 mg of elemental calcium per tablet. It is advisable to take supplements containing calcium alone (e.g., calcium carbonate) with meals to promote absorption of the calcium. SOURCE: Adapted from IOM (1992). |
|
trimester and less than 10.5 g/dl in the second trimester. The routine use of ferrous iron at a dose of about 30 rag/day was recommended for nonanemic women starting at 12 weeks of gestation.1
Anemia before conception or during pregnancy should be treated with 60 to 120 mg of ferrous iron per day, no more than about 60 mg per dose. A vitamin-mineral supplement containing 15 mg of zinc and 2 mg of copper was recommended to be taken at a different time of the day.2 The hemoglobin should be checked for improvement after about 1 month, and the dose should be lowered to 30 mg/day if the anemia has resolved. The possibility of side effects was mentioned, including the likelihood that nausea, cramps, constipation, or diarrhea, should they develop, often persist for no longer than 3 to 5 days. Liquid and chewable preparations were offered as alternatives for women who have trouble swallowing pills or capsules. The relevant information provided about iron supplements is summarized in Table B-13.
Life Research Office: Guidelines For The Assessment And Management Of Iron Deficiency In Women Of Childbearing Age
This report (LSRO, 1991) focused primarily on the problem of iron deficiency in nonpregnant women. The recommendations for pregnant women were essentially the same as those of the Institute of Medicine (IOM, 1990a, 1992).
- The prevalence of iron deficiency in the total population was considered to be sufficiently low to preclude recommending an increase in current levels of iron fortification in standardized food.
- The prevalence of iron deficiency in nonpregnant women of childbearing age was too low to justify supplementing all women. Women with iron deficiency anemia should therefore be detected by laboratory screening, and intervention with iron supplements should be recommended for women with iron deficiency anemia.
- Nonpregnant women should be screened for anemia (hemoglobin concentration, <12.0 g/dl, or hematocrit, <36 percent, with corrections for altitude and smoking [Table B-11]). For mild anemia (a hemoglobin concentration of between 10.0 and 12.0 g/all), a therapeutic dose of iron, 60 mg twice a day, should be given for 6 weeks. Check for a change in hemoglobin concentration and continue for a total of 6 months, and then lower the dose to 30 mg/day and monitor iron status. For severe anemia (a hemoglobin concentration of <10.0 g/dl), investigate further for the cause of anemia (note that such low values are very rare). To make an etiologic diagnosis of anemia, serum ferritin concentra
-
TABLE B-13 Information About Iron Supplements
|
Types of iron preparations |
|
|
|
|
|
|
Iron doses can be expressed in terms of elemental iron, as here, or in terms of the iron compound: |
|
|
|
What to tell the patient to improve compliance and safety |
|
|
|
|
|
|
SOURCE: IOM (1992). |
- tion determination is the preferred test. A value of less than 12 µg/liter by itself indicates iron deficiency. A value of less than 15 µg/liter in an anemic individual indicates iron deficiency anemia.
- Therapeutic doses of iron for anemia should be given under medical supervision. The total daily dose can be between 60 and 180 mg/day but should not be more than about 60 mg per dose. Take iron alone with water or fruit juice, not with milk, tea, or coffee.
- A maintenance dose of iron is 30 mg/day (range, 15 to 60 mg) when taken as prescribed (see discussion of RDAs below).
- Advise on diet.
- For follow-up, on a 6-week revisit, modify the dose according to the change in the hemoglobin concentration and compliance. Consider other causes of anemia if there has been no response.
- Pregnant women should routinely take a maintenance dose of iron in the second and third trimesters even if they are not anemic. If anemic, they should receive a therapeutic dose of iron as described below. New CDC criteria for anemia during pregnancy should be used (Table B-11) (CDC, 1989).
Recommended Dietary Allowances, 10th Edition
- For nonpregnant women, 15 mg of iron per day was considered to provide a sufficient margin of safety for essentially all adult women in the United States except for those with the most extreme menstrual losses, given usual dietary patterns (NRC, 1989). This is a reduction from 18 mg/day in the 1980 RDAs.
- For pregnant women, a daily increment of 15 mg/day (or a total of 30 mg/day) is recommended; this value is averaged over the entire pregnancy (NRC, 1989). Since the increased requirements of iron during pregnancy cannot be met by the iron content of the habitual diet of most Americans or the iron stores of at least some women, daily iron supplements are usually recommended.
Editorial Discussions
There is a widely held view in Britain (Hibbard, 1988) and elsewhere (Hemminki and Starfield, 1978) that no medication (including iron supplements) should be given to pregnant women in the absence of proven need. The view of Hemminki and coworkers (1989) was based on an earlier meta-analysis indicating no proven benefits of using vitamin or mineral supplements (Hemminki and Starfield, 1978). Hibbard (1988) proposed screening pregnant women and treating those in low-risk categories with iron only if they have a low serum ferritin concentration or anemia, but Horn (1988), in the same issue of the British Medical Journal as the article by Hibbard (1988), recommended
routine iron supplementation as practical and cost-effective. Hibbard (1988) also recommended routine iron administration if concomitant folic acid treatment was used. Thus, the recent recommendation to use folate before conception and early in pregnancy would also include the use of iron if iron and folate combinations are prescribed, as is commonly the case.
International Recommendations Directed Primarily to Developing Countries
These recommendations are intended primarily for pregnant women in developing countries, where surveys have shown a prevalence of iron deficiency anemia often in excess of 50 percent (DeMaeyer et al., 1985). The recommendations have involved the routine use of relatively high doses of iron and folate. Poor distribution of tablets and poor compliance have been recognized as major problems. Various strategies for fortifying food with iron have been studied, but there has been little progress in implementing them on a broad scale.
Fao/Who (1988)
Requirements for absorbed iron were calculated for various age, sex, and pregnancy categories. Recommendations for dietary iron intakes are based on the estimated bioavailability of iron from the customary diet. The assumed percentage of iron absorbed primarily from cereal and legume diets, which have poor iron bioavailability, are 5 percent; that absorbed from diets with intermediate bioavailability, 10 percent; and that absorbed from diets with high bioavailability (meat and ascorbic acid-rich diets, as is usual in the United States), 15 percent.
World Health Organization
The document Preventing and Controlling Iron Deficiency Anemia Through Primary Health Care (DeMaeyer, 1989) noted the need for supplementation in situations such as pregnancy, in which rapid improvement in iron status is important. It recognized the problem of compliance, especially when there may be side effects and no perception of ill health. It also stressed the need for communication and the importance of motivation skills for health workers. For pregnant women, the recommendation was to take tablets containing 60 mg of iron and 250 lag of folate twice a day. A diet rich in heme iron and ascorbic acid was advocated. The desirability of iron fortification of a staple food and the control of infection were discussed.
International Nutritional Anemia Consultative Group
Tablets containing 60 mg of iron and 250 µg of folate twice a day without food were recommended for pregnant women. If there were side effects, the tablets were to be taken after meals or at a lower dose for 1 week before resuming a full dose. Diet modification and fortification were mentioned (INACG, 1989).
References
AAP, CON (American Academy of Pediatrics, Committee on Nutrition). 1969. Iron balance and requirements in infancy. Pediatrics 43:134-142.
AAP, CON (American Academy of Pediatrics, Committee on Nutrition). 1976. Iron supplementation for infants. Pediatrics 58:765-768.
AAP, CON (American Academy of Pediatrics, Committee on Nutrition). 1985. Nutritional needs of low-birth-weight infants. Pediatrics 75:976-986.
AAP, CON (American Academy of Pediatrics, Committee on Nutrition). 1989. Iron-fortified infant formulas. Pediatrics 84:1114-1115.
AAP, CON (American Academy of Pediatrics, Committee on Nutrition). 1992. The use of whole cows milk in infancy. Pediatrics 89:1105-1109.
AAP, CON (American Academy of Pediatrics, Committee on Nutrition). 1993. Pediatric Nutrition Handbook. Elk Grove, Ill: AAP.
ACOG (American College of Obstetricians and Gynecologists). 1989. Committee on Professional Standards. Standards for Obstetric-Gynecological Services, 7th ed. Washington, D.C.: ACOG.
AMA (American Medical Association). 1992. Guidelines for Adolescent Preventive Services. Report of the Department of Adolescent Health. Chicago: AMA.
Annest, J.L., J.L. Pirckle, D. Makue, J.W. Neese, D.D. Bayse, and M.G. Kovar. 1983. Chronological trend in blood lead levels between 1976 and 1980. N. Engl. J. Med. 308:1373-1377.
Babior, B.M., W.A. Peters, P.M. Briden, and C.L. Cetrulo. 1985. Pregnant women's absorption of iron from prenatal supplements. J. Reprod. Med. 30:355-357.
Beutler, E. 1988. The common anemias. J. Am. Med. Assoc. 259:2433-2437.
Bonnar, J., A. Goldberg, and J.A. Smith. 1969. Do pregnant women take their iron? Lancet 1:457-458.
Bothwell, T.H., and R.W. Charlton. 1981. Iron Deficiency in Women. Washington, D.C.: International Nutrition Anemia Consultative Group.
Bothwell, T.H., and P. Macphail. 1992. Prevention of iron deficiency by food fortification. Pp. 183-192 in Nutritional Anemias, S.J. Fomon and S. Zlotkin, eds. Nestlé Nutrition Workshop Series, Vol. 30. New York: Raven Press.
Bothwell, T.H., R.W. Charlton, J.D. Cook, and C.A. Finch. 1979. Iron Metabolism in Man. Blackwell: Oxford.
Bothwell, T.H., R.D. Baynes, B.J. MacFarlane, and A.P. MacPhail. 1989. Nutritional iron requirements and food iron absorption. J. Int. Med. 226:357-365.
Calvo, E.B., A.C. Golindo, and N.B. Aspres. 1992. Iron status in exclusively breastfed infants. Pediatrics 90:375-379.
Canadian Paediatric Society, Nutrition Committee. 1991. Meeting the iron needs of infants and young children: An update. Can. Med. Assoc. J. 144:1451-1453.
Carriaga, M.T., B.S. Skikne, B. Finley, B. Cutler, and J.D. Cook. 1991. Serum transferrin receptor for the detection of iron deficiency in pregnancy. Am. J. Clin. Nutr. 54:1077-1081.
CDC (Centers for Disease Control). 1989. CDC criteria for anemia in children and childbearing-aged women. Morbid. Mortal. Weekly Rep. 38:400-404.
CDC (Centers for Disease Control). 1992. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. Morbid. Mortal. Weekly Rep. 41 (No. RR-14 ): 1-7.
Chanarin, I., and D. Rothman. 1971. Further observations on the relation between iron and folate status in pregnancy. Br. Med. J. 2:81-84.
Charlton, R.W., and T.H. Bothwell. 1983. Iron absorption. Annu. Rev. Meal. 34:55-68.
Clapp, J.F., B.L. Seaward, R.H. Sleamaker, and J. Hiser. 1988. Maternal physiologic adaptations to early pregnancy. Am. J. Obstet Gynecol. 159:1456-1460.
Clark, M., R. Royal, and R. Seeler. 1988. Interaction of iron deficiency and lead and the hematologic findings in children with severe lead poisoning. Pediatrics 81:247-254.
Cook, J.D. 1990. Adaptation in iron metabolism. Am. J. Clin. Nutr. 51:301-308.
Cook, J.D., and T.H. Bothwell. 1984. Availability of iron from infant food. Pp. 119-143 in Nutrition in Infancy and Childhood, A. Stekel, ed. New York: Raven Press.
Cook, J.D., C.A. Finch, and N.J. Smith. 1976. Evaluation of the iron status of a population. Blood 48:449-455.
Cook, J.D., S.S. Watson, K.M. Simpson, D.A. Lipschitz, and B.S. Skigne. 1984. The effect of high ascorbic acid supplementation on body iron stores. Blood 64:721-726.
Cook, J.D., S.A. Dassenko, and S.R. Lynch. 1991. Assessment of the role of nonheine-iron availability in iron balance. Am. J. Clin. Nutr. 54:717-722.
Cook, J.D., B.S. Skikme, and R.D. Baynes. 1993. Serum transferrin receptor. Annu. Rev. Med. 44:63-74.
Cramer, J.A., R.H. Mattson, M.L. Prevey, R.D. Scheyer, and V.L. Ouellette. 1989. How often is medication taken as prescribed? A novel assessment technique. J. Am. Med. Assoc. 261:3273-3277.
Cramer, J.A., R.D. Scheyer, and R.H. Mattson. 1990. Compliance declines between clinic visits. Arch. Intern. Med. 150:1509-1510.
Crosby, W.H. 1986. Yin, yang, and iron. Nutrition Today July/Aug:14-16.
Cross, C.E., B. Halliwell, E.T. Borish, W.A. Pryor, R.L. Saul, J.M. McDord, and D. Harman. 1987. Oxygen radicals and human disease. Ann. Intern. Med. 107:526-545.
Czeizel, A.E., and I. Dudas. 1992. Prevention of the first occurrence of neural-robe defects by periconceptual vitamin supplementation. N. Engl. J. Med. 327:1832-1835.
Dallman, P.R. 1986. Biochemical basis for manifestations of iron deficiency. Annu. Rev. Nutr. 6:13-40.
Dallman, P.R. 1987. Iron deficiency and the immune response. Am. J. Clin. Nutr. 46:329-334.
Dallman, P.R. 1988. Nutritional anemia of infancy: Iron, folic acid, and vitamin B12. Pp. 216-235 in Nutrition During Infancy, R.C. Tsang and B.L. Nichols, eds. Philadelphia: Hanley and Belfus.
Dallman, P.R. 1992. Changing iron needs from birth through adolescence. Pp. 29-36 in Nutritional Anemias, S.J. Fomon and S. Zlotkin, eds. New York: Raven Press.
Dallman, P.R., and J.D. Reeves. 1984. Laboratory diagnosis of iron deficiency. Pp. 11-44 in Nutrition in Infancy and Childhood, A. Stekel, ed. New York: Raven Press.
Dallman, P.R., and M.A. Siimes. 1979. Percentile curves for hemoglobin and red cell volume in infancy and childhood. J. Pediatr. 94:26-31.
Dallman, P.R., G.D. Bart, C.M. Allen, and H.R. Shinefield. 1978. Hemoglobin concentration in white, black, and Oriental children: is there a need for separate criteria in screening for anemia? Am. J. Clin. Nutr. 31:377-380.
Dawson, E.B., and W.J. McGanity. 1987. Protection of maternal iron stores in pregnancy. J. Reprod. Med. 32:478-487.
DeMaeyer, E.M. 1989. Preventing and Controlling Iron Deficiency Anaemia Through Primary Health Care. Geneva: World Health Organization.
DeMaeyer, E.M., M. Adiels-Tegman, and E. Rayston. 1985. The prevalence of anemia in the world. World Health Statist. Q. 38:302-316.
DHHS/USDA (U.S. Department of Health and Human Services and U.S. Department of Agriculture). 1991. Nutrition and Your Health: Dietary Guidelines for Americans, 3rd ed. Washington, D.C.: U.S. Government Printing Office.
Duncan, B., R.B. Schifman, J.J. Corrigan, and C. Schaefer. 1985. Iron and the exclusively breastfed infant from birth to six months. J. Pediatr. Gastroenterol. Nutr. 4:421-425.
Ekenved, G., B. Arvidsson, and L. Sölvell. 1976. Influence of food on the absorption from different types of iron tablets. Scand. J. Haematol. 28(Suppl.):79-88.
Expert Scientific Working Group. 1985. Summary of a report on assessment of the iron nutritional status of the United States population. Am. J. Clin. Nutr. 42:1318-1330.
FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization). 1988. 1988 Report of a Joint FAO/WHO Expert Consultation. Pp. 33-50 in Requirements of Vitamin A, Iron, Folate and Vitamin B12. Rome: Food and Agriculture Organization of the United Nations.
Ferguson, B.J., B.S. Skigne, K.M. Simpson, R.D. Baynes, and J.D. Cook. 1992. Serum transferrin receptor distinguishes the anemia of chronic from iron deficiency anemia. J. Lab. Clin. Meal. 119:385-390.
Fomon, S.J. 1987. Reflections on infant feeding in the 1970s and 1980s. Am. J. Clin. Nutr. 46:171-182.
Fomon, S.J., E.E. Ziegler, R.R. Rogers, S.E. Nelson, B.B. Edwards, D.G. Guy, J.C. Erve, and M. Janghorbani. 1989. Iron absorption from infant foods. Pediatr. Res. 26:250-254.
Galan, P., F. Cherouvrier, P. Preziosi, and S. Hercberg. 1991. Effects of the increased consumption of dairy products upon iron absorption. Eur. J. Clin. Nutr. 45:553-559.
Gain, S.M., S.A. Ridella, A.S. Tetzold, and F. Falkner. 1981. Maternal hematological levels and pregnancy outcomes. Semin. Perinatol. 5:155-162.
Groner, J.A., N.A. Holtzman, E. Charney, and D.E. Mellits. 1986. A randomized trial of oral iron on tests of short-term memory and attention span in young pregnant women. J. Adolescent Health Care 7:44-48.
Hahn, P.F., E.L. Carothers, W.J. Darby, M. Martin, C.W. Sheppard, R.O. Cannon, A.S. Beam, P.M. Denson, J.C. Peterson, and G.S. McClellan. 1951. Iron metabolism in human pregnancy as studied with the radioactive isotope, Fe59 . Am. J. Obstet. Gynecol. 61:477-486.
Hallberg, L. 1981. Bioavailability of dietary iron in man. Annu. Rev. Nutr. 1:123-147.
Hallberg, L. 1992. Iron requirements. Comments on methods and some crucial concepts in iron nutrition. Biol. Trace Elements Res. 35:25-45.
Hallberg, L. 1988. Iron balance in pregnancy. Pp 115-126 in Vitamins and Minerals in Pregnancy and Lactation, H. Berger, ed. Nestlé Nutrition Workshop Series, Vol. 16. New York: Raven Press.
Hallberg, L., and L. Rossander. 1982. Absorption of iron from Western-type lunch and dinner meals. Am. J. Clin. Nutr. 35:502-509.
Hallberg, L., A.M. Högdahl, L. Nilsson, and G. Rybo. 1966. Menstrual blood loss—a population study. Variation at different ages and attempts to define normality. Acta Obstet. Gynecol. Scand. 45:320-351.
Hallberg, L., L. Ryttinger, and L. Sölvell. 1967. Side effects of oral iron therapy: A double-blind study of different iron compounds in tablet form . Acta Med. Scand. Suppl. 459:3-10.
Hallberg, L., M. Brune, M. Erlandsson, A.-S. Sandberg, and L. Rossander-Hultén. 1991. Calcium: Effect of different amounts on non-heme- and heine-iron absorption in humans. Am. J. Clin. Nutr. 53:112-119.
Hallberg, L., L. Rossander-Hulten, M. Brune, and A. Gleerup. 1992. Bioavailability of iron in human milk and cow's milk in relation to their calcium contents. Pediatr. Res. 31:524-527.
Hallberg, L., L. Hulten, G. Lindstedt, P.-A. Lunberg, M. Anders, J. Purens, B. Svanberg, and B. Swolin. In press. High prevalence of iron deficiency in Swedish adolescents. Pediatr. Res.
Hambidge, K.M., N.F. Krebs, L. Sibley, and J. English. 1987. Acute effects of iron therapy on zinc status during pregnancy. Obstet. Gynecol. 70:593-596.
Haynes, R.B. 1979. Strategies for improving compliance: A methodologic analysis and review. Pp 69-82 in Compliance in Health Care, R.B. Haynes, D.W. Taylor, and D.L. Sackett, eds. Baltimore: Johns Hopkins University Press.
Haynes, R.B., D.W. Taylor, and D.L. Sackett, eds. 1979. Compliance in Health Care. Baltimore: Johns Hopkins University Press.
Hemminki, E., and B. Starfield. 1978. Routine administration of iron and vitamins during pregnancy: Review of controlled clinical trials. Br. J. Obstet. Gynaecol. 85:404-410.
Hibbard, B.M. 1988. Controversies in therapeutics. Iron and folate supplements during pregnancy: Supplementation is valuable only in selected patients. Br. Med. J. 297:1324-1326.
Horn, E. 1988. Controversies in therapeutics. Iron and folate supplements during pregnancy: Supplementing everyone treats those at risk and is cost effective. Br. Med. J. 297:1325-1327.
Hurrell, R.F. 1992. Prospects for improving the iron fortification of foods. Pp. 193-201 in Nutritional Anemias, S.J. Fomon and S. Zlotkin, eds. Nestlé Nutrition Workshop Series, Vol. 30. New York: Raven Press.
Idjradinata, P., and E. Pollitt. 1993. Reversal of developmental delays in iron deficient anaemic infants treated with iron. Lancet 341:1-4.
INACG (International Nutritional Anemia Consultative Group). 1989. Guidelines for the Control of Maternal Nutritional Anemia. International Life Sciences Institute-Nutrition Foundation. Washington, D.C.: INACG.
IOM (Institute of Medicine). 1990a. Nutrition During Pregnancy. Report of the Committee on Nutritional Status During Pregnancy and Lactation, Food and Nutrition Board. Washington, D.C.: National Academy Press.
IOM (Institute of Medicine). 1990b. Iron nutrition during pregnancy. Pp. 272-298 in Nutrition During Pregnancy. Report of the Committee on Nutritional Status During Pregnancy and Lactation, Food and Nutrition Board. Washington, D.C.: National Academy Press.
IOM (Institute of Medicine). 1992. Nutrition During Pregnancy and Lactation: An Implementation Guide. Report of the Subcommittee for a Clinical Application Guide, Committee on Nutritional Status During Pregnancy and Lactation, Food and Nutrition Board. Washington, D.C.: National Academy Press.
Johnson-Spear, M.A., and R. Yip. In press. Hemoglobin difference between black and white women with comparable iron status: Justification for race-specific criteria? Am. J. Clin. Nutr.
Kastrup, E.K., ed. 1992. Drug Facts and Comparisons. St. Louis: Facts and Comparisons.
Kim, I., D.W. Hungerford, R. Yip, S.A. Kuester, C. Zyrkowski, and F.L. Trowbridge. 1992. Pregnancy nutrition surveillance system—United States, 1979-1990. CDC Surveillance Summaries. Morbid. Mortal. Weekly Rep. 41(No. SS-7):26-42.
Laplan, J.P., J.L. Annest, P.M. Layde, and G.L. Rubin. 1986. Nutrient intake and supplementation in the United States (NHANES II). Am. J. Public Health 76:287-289.
Litovitz, T.L., K.C. Holm, K.M. Bailey, and B.F. Schmitz. 1992. 1991 Annual report of the American Association of Poison Control Centers National Data Collection System. Am. J. Emerg. Med. 10:452-505.
Looker, A.C., C. L. Johnson, M.A. McDowell, and E.A. Yetley. 1989. Iron status: Prevalence of impairment in three Hispanic groups in the United States. Am. J. Clin. Nutr. 49:553-558.
Lozoff, B., E. Jimenez, and A.B. Wolf. 1991. Long-term developmental outcome of infants with iron deficiency. N. Engl. J. Med. 325:687-694.
LSRO (Life Sciences Research Office). 1984. Assessment of the Iron Nutritional Status of the U.S. Population Based on Data Collected in the Second National Health and Nutrition Examination Survey, 1976-1980. Bethesda, Md.: Federation of American Societies for Experimental Biology.
LSRO (Life Sciences Research Office). 1989. Nutrition Monitoring in the United States—An Update Report on Nutrition Monitoring. DHHS Publication No. (PHS) 89-1255. Public Health Service. Washington, D.C.: U.S. Government Printing Office.
LSRO (Life Sciences Research Office). 1991. Guidelines for the Assessment and Management of Iron Deficiency in Women of Childbearing Age, S.A. Anderson, ed. Bethesda, Md.: Federation of American Societies for Experimental Biology.
Lundström, U., M.A. Siimes, and P.R. Dallman. 1977. At what age does iron supplementation become necessary in low-birth-weight infants? J. Pediatr. 91:878-883.
Monsen, E.R., L. Hallberg, M. Layrisse, D.M. Hegsted, J.D. Cook, W. Mertz, and C.A. Finch. 1978. Estimation of available dietary iron. Am. J. Clin. Nutr. 31:134-141.
Murphy, J.F., J. O'Riordan, R.G. Newcombe, and E.C. Coles. 1986. Relation of hemoglobin levels in first and second trimesters to outcome of pregnancy. Lancet 1:992-994.
NRC (National Research Council). 1989. Recommended Dietary Allowances, 10th ed. Report of the Subcommittee on the Tenth Edition of the RDAs, Food and Nutrition Board, Commission of Life Sciences. Washington, D.C.: National Academy Press.
Penrod, J.C., K. Anderson, and P.B. Acosta. 1990. Impact on iron status of introducing cow's milk in the second six months of life. J. Pediatr. Gastroenterol. Nutr. 10:462-467.
Perry, G.S., T. Byers, R. Yip, and S. Margen. 1992. Iron nutrition does not account for hemoglobin differences between blacks and whites. J. Nutr. 122:1417-1424.
Pizarro, F., R. Yip, P.R. Dallman, M. Olivares, E. Hertrampf, and R. Walter. 1991. Iron status with different infant feeding regimens: Relevance to screening and prevention of iron deficiency. J. Pediatr. 118:687-692.
Pollitt, E., P. Hathirat, N.J. Kotchabhakdi, L. Missell, and A. Valyasevi. 1989. Iron deficiency and educational achievement in Thailand. Am. J. Clin. Nutr. 50:687-697.
Porter, A.M.W. 1969. Drug defaulting in a general practice. Br. Med. J. 1:218-222.
Puolakka, J., O. Jãnne, A. Pakarinen, and R. Vihko. 1980. Serum ferritin as a measure of stores during and after normal pregnancy with and without iron supplements. Acta Obstet. Gynecol Scand. Suppl. 95:43-51.
Reeves, J.D., R. Yip, V. Kiley, and P.R. Dallman. 1984. Iron deficiency in infants, the influence of antecedent infection. J. Pediatr. 105:874-879.
Romslo, I., K. Haram, N. Sagen, and K. Augensen. 1983. Iron requirements in normal pregnancy as assessed by serum ferritin, serum transferrin saturation, and erythrocyte protoporphyrin determinations. Br. J. Obstet. Gynaecol. 90:101-107.
Rossander, L., L. Hallberg, and E. Björn-Rasmussen. 1979. Absorption of iron from breakfast meals. Am. J. Clin. Nutr. 32:2484-2489.
Salonen, J.T., K. Nyysönen, H. Korpela, J. Tuomilehto, R. Seppänen, and R. Salonen. 1992. High stored iron levels are associated with excess risk of myocardial infarction in Eastern Finnish men . Circulation 86:803-811.
Schifman, R.B., J.E. Thomasson, and J.M. Evers. 1987. Red blood cell zinc protoporphyrin testing for iron deficiency anemia in pregnancy. Am. J. Obstet. Gynecol. 157:304-307.
Scholl, T.O., M.L. Hediger, R.L. Fischer, and J.W. Shearer. 1992. Anemia vs. iron deficiency: Increased risk of preterm delivery in a prospective study. Am. J. Clin. Nutr. 55:985-988.
Seligman, P.A., J.H. Caskey, J.L. Frazier, R.M. Zucker, E.R. Podell, and R.H. Alien. 1983. Measurements of iron absorption from prenatal multivitamin-mineral supplements. Obstet. Gynecol. 61:356-362.
Sheldon, W.L., M.O. Aspillaga, P.A. Smith, and T. Lind. 1985. The effects of oral iron supplementation on zinc and magnesium levels during pregnancy. Br. J. Obstet. Gynaecol. 92:892-898.
Siegenberg, D., R.D. Baynes, T.H. Bothwell, B.J. MacFarlane, R.D. Lamparelli, N.G. Car, P. McPhail, U. Schmidt, A. Tal, and F. Mayet. 1991. Ascorbic acid prevents the dose dependent inhibitory effects of polyphenols and phytates on non-heme iron absorption. Am. J. Clin. Nutr. 53:537-541.
Siimes, M.A., L. Salmenperä, and J. Perheentupa. 1984. Exclusively breast-feeding for nine months: Risk of iron deficiency. J. Pediatr. 104:196-199.
Sjöstedt, J.E., P. Manner, S. Nummi, and G. Ekenved. 1977. Oral iron prophylaxis during pregnancy: A comparative study on different dosage regimens. Acta Obstet. Gynecol. Scand. Suppl. 60:3-9.
Sölvell, L. 1970. Oral iron therapy-side effects. Pp. 573-583 in Iron Deficiency: Pathogenesis, Clinical Aspects, Therapy, L. Hallberg, H.G. Harwerth, and A. Vannotti, eds. London: Academic Press.
Subar, A.F., and G. Block. 1990. Use of vitamin and mineral supplements: Demographics and amounts of nutrients consumed. Am J. Epidemiol. 132:1091-1101.
Svanberg, B., B. Arvidsson, A. Norrby, G. Rybo, and L. Sölvell. 1976. Absorption of supplemental iron during pregnancy—A longitudinal study with repeated
bone-marrow studies and absorption measurements. Acta Obstet. Gynecol. Scand Suppl. 48:87-108.
Swiss, L.D., and G.H. Beaton. 1974. A prediction of the effects of iron fortification. Am. J. Clin. Nutr. 27:373-379.
Taylor, D.J., C. Mallen, N. McDougall, and T. Lind. 1982. Effect of iron supplementation on serum ferritin levels during and after pregnancy. Br. J. Obstet. Gynecol. 89:1011-1017.
Tunnessen, W.W., and F.A. Oski. 1987. Consequences of starting whole cow milk at 6 months of age. J. Pediatr. 111:813-816.
Uchida, T., M. Yoshida, K. Sakai, K. Kokubun, T. Igarashi, T. Tanaka, and S. Kariyone. 1988. Prevalence of iron deficiency in Japanese women. Acta Haematol. Jpn. 51:24-27.
Ulmer, H.U., and E. Goepel. 1988. Anemia, ferritin and preterm labor. J. Perinat. Med. 16:459-465.
Wallenburg, H.C.S., and H.G. van Eijk. 1984. Effect of oral iron supplementation during pregnancy on maternal and fetal iron status. J. Perinat. Med. 12:7-11.
Walter, T., I. De Andraca, P. Chadud, and C.G. Perales. 1989. Iron deficiency anemia: Adverse effects on infant psychomotor development. Pediatrics 84:7-17.
Walter, T., P.R. Dallman, F. Pizarro, L. Veloso, G. Peña, S. Bartholmey, E. Hertrampf, M. Olivares, A. Letelier, and M. Arredondo. 1993. Effectiveness of iron-fortified infant cereal in prevention of iron deficiency anemia. Pediatrics 91:976-982.
Weinberg, E.D. 1984. Iron withholding. Physiol. Rev. 64:65-102.
Weinberg, E.D. 1992. Roles of iron in neoplasia. Biol. Trace Element Res. 34:123-140.
Widholm, O., E. Vartiainen, and T. Tenhunen. 1967. On iron requirements in menstruating teen-age girls. Acta Obstet. Gynecol. Scand. 46(Suppl. 1):31-46.
Yip, R. 1989. The changing characteristics of childhood iron nutritional status in the United States. Pp. 37-56 in Dietary Iron: Birth to Two Years, L.J. Filer, Jr., ed. New York: Raven Press.
Yip, R., S. Schwartz, and A.S. Deinard. 1984. Hematocrit values in white, black, and American Indian children with comparable iron status: Evidence to support uniform diagnostic criteria for anemia among all races. Am. J. Dis. Child. 138:824-827.
Yip, R., K.M. Walsh, M.G. Goldfarb, and N.J. Binkin. 1987a. Declining prevalence of anemia in childhood in a middle-class setting: A pediatric success story? Pediatrics 80:330-334.
Yip, R., N.J. Binkin, L. Fleshood, and F.L. Trowbridge. 1987b. Declining prevalence of anemia among low-income children in the United States. J. Am. Med. Assoc. 258:1619-1623.
Ziegler, E.E., S.J. Fomon, S.E. Nelson, C.J. Rebouche, B.B. Edwards, R.R. Rogers, and L.J. Lehman. 1990. Cow milk feeding in infancy: Further observations on blood loss from the gastrointestinal tract. J. Pediatr. I 16:11-18.


























































